
Full Answer
Are there side effects of monoclonal antibody treatment?
Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous (IV) infusion. Another option for COVID-19 therapy is an antiviral called Remdesivir. Remdesivir is approved by the FDA and helps reduce the effects of COVID-19. Remdesivir is given by an intravenous (IV) infusion over three (3) consecutive days.
How effective is the monoclonal treatment?
Infusion_Units_for_COVID-19_Antibody_Treatment_Operations_Guide.pdf The following summary can help you prepare your site to administer monoclonal antibody treatment. Prepare* *Infusion locations should consider all local and state requirements. English: CombatCOVID.hhs.gov • 1-877-332-6585 Spanish: CombateCOVID.hhs.gov • 1-877-366-0310 04/09/21
What are the dangers of monoclonal antibodies?
Depending on the mAb treatment you receive, the whole process takes about 1-3 hours. First, the medical staff conduct a health screening; then they start an IV, which delivers the mAbs to your body in just over an hour. It takes less time if the mAb treatment is offered to …
When to administer monoclonal antibodies?
Consistent with existing payment methodologies for the care setting where you provide the treatment; For COVID-19 monoclonal antibody products administered before May 6, 2021, the Medicare payment rate is approximately $310. Medicare will establish codes and rates for administering new products as the FDA approves or authorizes each product.
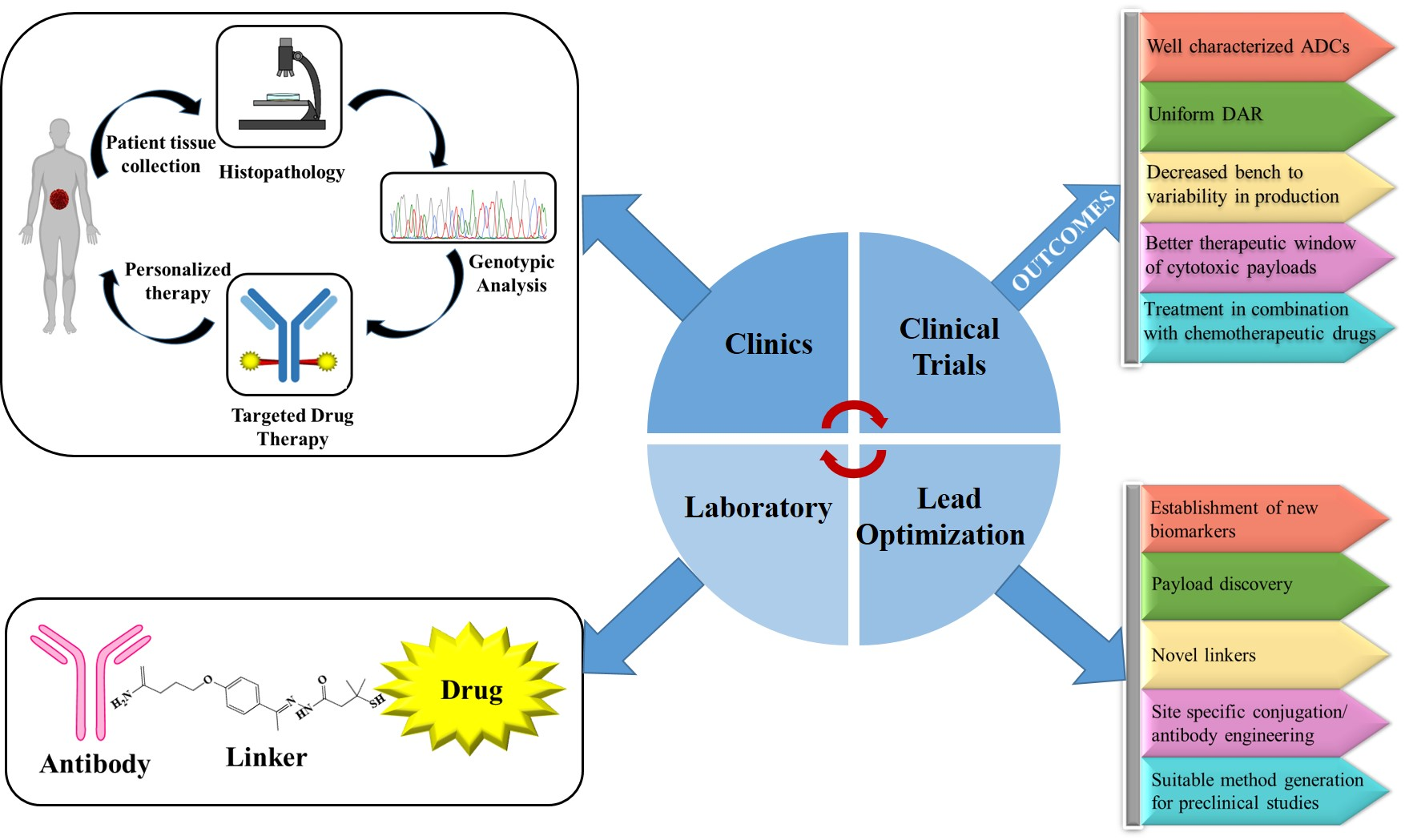
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Who could benefit from monoclonal antibody therapy to prevent COVID-19?
See full answerVaccines are the best way to protect against COVID-19. But some people with weakened immune systems do not produce enough antibodies after vaccination, and others are severely allergic to the vaccine. The FDA recently authorized Evusheld, a pre-exposure prophylaxis (PrEP) monoclonal antibody therapy developed by AstraZeneca, which should help prevent COVID-19 in these populations.To be eligible for Evusheld, individuals must be 12 years or older and have a moderately to severely weakened immune system, or have a history of severe adverse reactions to the COVID-19 vaccine or its components. In addition, the therapy cannot be given to someone with a current SARS-CoV-2 infection, or who has been recently exposed to someone who is infected. Evusheld is given as two consecutive shots, and evidence suggests it can help prevent symptomatic infection for at least six months.Apr 1, 2022
What is a monoclonal antibody for COVID-19?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells. Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
Is there a monoclonal antibody therapy for post COVID-19 exposure?
FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19 | FDA.Sep 16, 2021
Are antibodies beneficial during the COVID-19 pandemic?
When reinfections or breakthrough infections happen, having antibodies plays an important role in helping prevent severe illness, hospitalization, and death. For many diseases, including COVID-19, antibodies are expected to decrease or “wane” over time.Nov 10, 2021
What is a monoclonal antibody?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
Is there an antibody cocktail for COVID-19?
The treatment, bamlanivimab and etesevimab administered together, was granted FDA emergency use authorization in February. Eli Lilly and the FDA stipulated that the antibody cocktail is authorized as a COVID-19 prophylaxis only for individuals who have been exposed to the virus.Sep 16, 2021
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
Can I get COVID-19 again after having the vaccine?
Getting COVID-19 after you've been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you.Nov 10, 2021
Who should not take the Pfizer-BioNTech COVID-19 vaccine?
If you have had a severe allergic reaction to any ingredient in the Pfizer-BioNTech COVID-19 vaccine (such as polyethylene glycol), you should not get this vaccine. If you had a severe allergic reaction after getting a dose of the Pfizer-BioNTech COVID-19 vaccine, you should not get another dose of an mRNA vaccine.
How do monoclonal antibodies work against cancer?
Monoclonal antibodies are immune system proteins that are created in the lab. Antibodies are produced naturally by your body and help the immune sy...
Which cancers are treated with monoclonal antibodies?
Many monoclonal antibodies have been approved to treat a wide variety of cancers. To learn about specific treatments for your cancer, see the PDQ®...
What are the side effects of monoclonal antibodies?
Monoclonal antibodies can cause side effects, which can differ from person to person. The ones you may have and how they make you feel will depend...
How long does it take for a virus to develop antibodies?
A vaccine triggers your body’s natural immune response, but can take weeks to develop enough antibodies and prevent some kinds of infection. Some vaccines for COVID-19 require two shots, so your body can develop its own immune response to the disease.
What is mAb treatment?
It’s called monoclonal antibody (mAb) treatment. Some early evidence suggests that mAb treatment can reduce the amount of the SARS-CoV-2 virus (the virus that causes COVID-19) in a person's system. This amount is known as viral load.
How long does it take for mAbs to get into your body?
First, medical staff conduct a screening; then they start an IV, which delivers the mAbs to your body in just over an hour. Afterward, the medical staff will have you stay at the infusion center for another hour to be sure you aren’t having an allergic reaction or other side effects.
How long do you have to be isolated from a virus?
It’s important to know that even if you start feeling better, you could still spread the virus for a while. So, you’ll need to isolate yourself (be alone) until all of these things happen: 1 At least 10 days have passed since your first symptoms of COVID-19 2 You haven’t had a fever in at least 24 hours, without taking any medicine that reduces fever 3 Your other symptoms of COVID-19 are improving
Can antibody infusion cause swelling?
An infusion of any medicine may cause brief pain, bleeding, bruising of the skin, soreness, swelling, and possible infection at the infusion site. These are not all the possible side effects of antibody treatment. Serious and unexpected side effects may happen. Some possible risks from antibody treatment are:
COVID-19 VEKLURYTM (remdesivir)
Following the recent statement from the National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel about therapies for the COVID-19 Omicron variant, CMS created HCPCS code J0248 for VEKLURY™ (remdesivir) antiviral medication when administered in an outpatient setting.
COVID-19 Monoclonal Antibody Products
The FDA authorized the following investigational monoclonal antibody product under EUA for pre-exposure prophylaxis of COVID-19:
Important Update about Viral Variants
On April 16, 2021, the FDA revoked the EUA for bamlanivimab, when administered alone , due to a sustained increase in COVID-19 viral variants in the U.S. that are resistant to the solo product.
Medicare Coverage for COVID-19 Monoclonal Antibody Products
During the COVID-19 public health emergency (PHE), Medicare will cover and pay for these infusions (when furnished consistent with their respective EUAs) the same way it covers and pays for COVID-19 vaccines.
Coding for the Administration of COVID-19 Monoclonal Antibody Products
CMS identified specific code (s) for each COVID-19 monoclonal antibody product and specific administration code (s) for Medicare payment:
Medicare Payment for Administering COVID-19 Monoclonal Antibody Products
To ensure immediate access during the COVID-19 PHE, Medicare covers and pays for these infusions and injections in accordance with Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) .
Billing for Administering COVID-19 Monoclonal Antibody Products
Health care providers can bill on a single claim for administering COVID-19 monoclonal antibody products, or submit claims on a roster bill.
Why are monoclonal antibodies used in immunotherapy?
Some monoclonal antibodies are also immunotherapy because they help turn the immune system against cancer. For example, some monoclonal antibodies mark cancer cells so that the immune system will better recognize and destroy them.
What is monoclonal antibody?
Monoclonal antibodies are immune system proteins that are created in the lab. Antibodies are produced naturally by your body and help the immune system recognize germs that cause disease, such as bacteria and viruses, and mark them for destruction.
What antibodies kill cancer cells?
Other monoclonal antibodies bring T cells close to cancer cells, helping the immune cells kill the cancer cells. An example is blinatumomab (Blincyto®), which binds to both CD19, a protein found on the surface of leukemia cells, and CD3, a protein on the surface of T cells. This process helps the T cells get close enough to ...
Can cytokine release cause shock?
Capillary leak syndrome may lead to multiple organ failure and shock. Cytokine release syndrome can sometimes occur with monoclonal antibodies, but it is often mild. Cytokines are immune substances that have many different functions in the body, and a sudden increase in their levels can cause: Fever. Nausea.
Can monoclonal antibodies cause side effects?
Monoclonal antibodies can cause side effects, which can differ from person to person. The ones you may have and how they make you feel will depend on many factors, such as how healthy you are before treatment, your type of cancer, how advanced it is, the type of monoclonal antibody you are receiving, and the dose.
What are monoclonal antibodies?
Antibodies are naturally produced by your body to fight off infections. When your body is introduced to a new virus such as COVID-19, it does not have the antibodies to fight it off. That is where monoclonal antibodies come in. Monoclonal antibodies are created in a laboratory. They can target a particular virus or infection such as COVID-19.
How does monoclonal antibody therapy work?
Monoclonal antibodies are given by IV or a single-dose injection to people diagnosed with COVID-19. This therapy uses COVID-19 antibodies to help a person’s body fight off the infection. The injection is a lower dosage than the infusion therapy.
What monoclonal antibody therapies for COVID-19 are available?
The Food and Drug Administration (FDA) has approved emergency use authorization for five antibody infusion therapies:
Is monoclonal antibody therapy effective against the Omicron variant?
So far, it appears only one of the monoclonal antibody treatments – sotrovimab – is effective against the Omicron variant for outpatient treatment. Most of the other monoclonal antibody treatments have limited or no effectiveness against the Omicron variant .
Who should get monoclonal antibody therapy?
Monoclonal antibody treatment is now available for three specific uses:
Who is at high risk for severe illness from COVID-19?
While anybody can get very sick or even die from COVID-19, those most at risk include:
What COVID-19 treatment is available for people diagnosed with COVID-19?
If you are diagnosed with COVID-19 but aren’t sick enough to be hospitalized, you may think there isn’t much you can do. It is important to:
What are monoclonal antibodies?
Monoclonal antibodies to fight COVID-19 are artificially manufactured antibodies designed to mimic your body’s natural antibodies.
Who is eligible for monoclonal antibodies?
Monoclonal antibody treatments are only available to certain patients.
How monoclonal antibodies are administered
Monoclonal antibodies are only given intravenously (through an IV) or as a subcutaneous injection (as a shot). That means that in order to receive them, you need to be seen in a medical setting — which limits the overall availability of the treatment.
How monoclonal antibodies compare to vaccination
If you’re not yet fully vaccinated when you receive monoclonal antibodies, you’ll have to wait 90 days to get the vaccine. Otherwise, the antibodies may impact the vaccine’s effectiveness.
