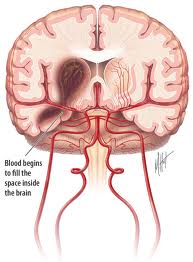
The traditional treatment of subarachnoid hemorrhage (SAH) from a ruptured cerebral aneurysm included strict blood pressure control, with fluid restriction and antihypertensive therapy. This approach was associated with a high rate of morbidity and mortality from the ischemic complications of hypovolemia and hypotension.
Full Answer
Are corticosteroids beneficial or harmful to patients with subarachnoid hemorrhage (SAH)?
Overall, there is no evidence of a beneficial or adverse effect of corticosteroids in patients with either SAH or PICH. Confidence intervals are wide and include clinically significant effects in both directions. Corticosteroids for aneurysmal subarachnoid haemorrhage and primary intracerebral haemorrhage Cochrane Database Syst Rev.
Should dexamethasone be used to treat subarachnoid and primary intracerebral haemorrhage?
Abstract Background: Corticosteroids, particularly dexamethasone, are commonly used for treatments in patients with subarachnoid haemorrhage (SAH) and primary intracerebral haemorrhage (PICH) despite the lack of evidence.
What is the initial management of intracranial hemorrhage (ICH)?
Emergency management ICH is a neurological emergency and initial management should be focused on assessing the patients airway, breathing capability, blood pressure and signs of increased intracranial pressure.
Is subarachnoid hemorrhage a medical emergency?
Subarachnoid hemorrhage is a medical emergency. Patients should call 9-1-1 and go to the nearest emergency room immediately.

What is the most important management strategy for intracerebral hemorrhage patients?
IVH and Hydrocephalus A number of strategies are available to manage IVH. The most common is the placement of an external ventricular drain (EVD), which may reduce intracranial pressure; however, this effect is counterbalanced by the risk of infection and catheter obstruction by clots [96, 98].
What is the primary goal of treatment for intracranial hemorrhage strokes?
The goals of initial treatment include preventing hemorrhage expansion, monitoring for and managing elevated intracranial pressure, and managing other neurologic and medical complications (table 1).
How is intracerebral haemorrhage treated?
Treatment. Treatment focusses on stopping the bleeding, removing the clot and relieving pressure on the brain. If left alone, the brain will eventually re-absorb the clot. The damage done by increased brain pressure over a long period may be irreversible.
What is the best treatment option for hemorrhagic stroke?
An injection of TPA is usually given through a vein in the arm within the first three hours. Sometimes, TPA can be given up to 4.5 hours after stroke symptoms started. This drug restores blood flow by dissolving the blood clot causing the stroke.
What is the treatment of hemorrhage?
Treating minor or mild hemorrhages typically involves rest and hydration. Typically, a clot will develop that temporarily limits bleeding while the blood vessel repairs itself. Over time, the surrounding bodily tissues will reabsorb the excess blood.
What medication is given to all aneurysmal subarachnoid hemorrhage patients to improve outcomes?
Nimodipine 60 mg every 4 hours given orally was well tolerated and reduced cerebral infarction. It also improved the outcome after subarachnoid hemorrhage.
What is subarachnoid haemorrhage?
A subarachnoid haemorrhage is an uncommon type of stroke caused by bleeding on the surface of the brain. It's a very serious condition and can be fatal.
Can intracerebral hemorrhage be cured?
How is intracerebral hemorrhage treated? Treatment within the first three hours of the onset of symptoms generally results in a better outcome. Surgery can relieve pressure on your brain and repair torn arteries. Certain medications can help manage symptoms, such as painkillers to ease severe headaches.
What monitoring tool might you expect for a patient with intracerebral hemorrhage?
Computed tomography (CT) is more widely available so CT of the brain has become the initial diagnostic test of choice for ICH. However, recent studies suggest MRI and CT are equally efficacious in diagnosing hyperacute ICH (<6 hours) (Fiebach et al 2004; Kidwell et al 2004).
What is the first aid treatment for stroke?
Three Things to Do When Someone Is Having a StrokeCall 911 immediately. ... Note the time you first see symptoms. ... Perform CPR, if necessary. ... Do not let that person go to sleep or talk you out of calling 911. ... Do not give them medication, food, or drinks. ... Do not drive yourself or someone else to the emergency room.More items...•
What are 3 treatments for a stroke?
Treating ischaemic strokesThrombolysis – "clot buster" medicine. ... Thrombectomy. ... Aspirin and other antiplatelets. ... Anticoagulants. ... Blood pressure medicines. ... Statins. ... Carotid endarterectomy.
What are the treatment of stroke?
The main treatment for ischemic stroke is intravenous tissue plasminogen activator (tPA), which breaks up clots. 2018 guidelines from the American Heart Association (AHA) and the American Stroke Association (ASA) state that tPA is most effective when it's given within four and a half hours from the start of a stroke.
What is a subarachnoid hemorrhage?
A subarachnoid hemorrhage is bleeding in the space around the brain. This bleeding between the thin layers of tissue that cover the brain often cau...
What is an intracranial hemorrhage?
Intracranial hemorrhage, also known as intracerebral hemorrhage, is a form of a stroke where there is bleeding on the brain.
What are causes of a subarachnoid hemorrhage?
If an intracranial aneurysm ruptures, it can result in a subarachnoid hemorrhage.
What are causes of an intracranial hemorrhage?
Intracranial hemorrhage may be caused by trauma, high blood pressure, or a vascular abnormality.
What are the symptoms of subarachnoid hemorrhages?
The most common symptom of subarachnoid hemorrhage is severe headache. This is frequently associated with nausea, stiff neck, and sensitivity to li...
What are the symptoms of intracranial hemorrhage?
Symptoms of an intracranial hemorrhage vary, but usually develop without warning. This type of brain bleed can cause dizziness, sudden severe heada...
How are subarachnoid hemorrhages diagnosed?
A computed tomography (CT or CAT) scan of the brain is used to diagnose the subarachnoid hemorrhage, and a cerebral angiogram is then performed to...
How is an intracranial hemorrhage diagnosed?
When diagnosing an intracranial hemorrhage, a neurosurgeon may find decreases in brain function and swelling of the optic nerve upon physical exami...
Is surgery necessary for a subarachnoid hemorrhage?
Subarachnoid hemorrhage is a medical emergency. Patients should call 9-1-1 and go to the nearest emergency room immediately. Patients with subarach...
Is surgery necessary for an intracranial hemorrhage?
An intracranial hemorrhage requires immediate care to reduce brain damage and prevent death. Treatment depends on the cause, location and extent of...
What is subarachnoid hemorrhage?
A subarachnoid hemorrhage is bleeding in the space around the brain. This bleeding between the thin layers of tissue that cover the brain often causes serious consequences such as death or severe disability.
Why do we need to do intracranial hemorrhage?
An intracranial hemorrhage requires immediate care to reduce brain damage and prevent death. Treatment depends on the cause, location and extent of bleeding. Brain surgery may be necessary to repair blood vessels or remove areas where blood has collected (hematomas).
What are the complications of subarachnoid hemorrhage?
Patients with subarachnoid hemorrhage require treatment for the aneurysm as well as treatment for complications of the hemorrhage such as vasospasm (constricting of a blood vessel which reduces blood flow and increases pressure) and hydrocephalus (fluid build-up in the brain).
What happens if an intracranial aneurysm ruptures?
If an intracranial aneurysm ruptures, it can result in a subarachnoid hemorrhage.
What is the term for a stroke where there is bleeding on the brain?
Intracranial hemorrhage , also known as intracerebral hemorrhage, is a form of a stroke where there is bleeding on the brain.
What is the procedure to diagnose intracranial hemorrhage?
Various blood tests may be conducted to determine the cause and amount of bleeding. Computed tomography (CT or CAT), magnetic resonance imaging (MRI) or angiography (in which dye is injected into an artery and viewed with an x-ray) may be performed to diagnose the location and extent of the bleeding.
What can be used instead of open surgery?
Sometimes less invasive neuroendovascular treatments can be used instead of open surgery. Medications may be prescribed to reduce swelling in the brain, reduce pain, and control seizures that can result from hemorrhaging. Intravenous infusion of medications, fluids and blood products may be necessary.
What is the next recommended diagnostic tool for SAH?
If non-contrast head CT is not definitive (time to study, patient elements [i.e., severe anemia], interpretation limitations [i.e., trainee radiologist, motion artifact], etc) the next recommended diagnostic tool is the LP. In these instances the LP is looking for two elements that raise the concern for SAH: 1) RBCs; and 2) xanthochromia (bilirubin in cerebrospinal fluid [CSF]).
What is CT for SAH?
When a clinical suspicion for SAH exists based on history and physical exam, non-contrast computed tomography (CT) is the first diagnostic tool. It is also valuable in excluding other pathologies such as intracranial hemorrhage, malignancy, or abscess.
Why are Hunt and Hess grades I and II patients more commonly missed?
As we delve into the diagnosis of SAH, it is important to note that some patients with SAH, for example Hunt and Hess grades I and II patients, are more commonly missed because symptoms are milder, and they may have smaller aneurysms with less subarachnoid blood. These patients do notnecessarily do better or have less morbidity with rupture or re-rupture.
How to detect xanthochromia?
Xanthochromia is detected either by visual inspection of the CSF tube vs a tube of water, or by spectrophotometry. RBCs that have shed into CSF from SAH will ultimately break down and release oxyhemoglobin, which then converts to bilirubin in vivo, interpreted as xanthochromia, or literally “yellow color.” It should be noted that blood from a traumatic tap can produce oxyhemoglobin when exposed to natural light, which can produce a yellow color, but since it is outside the body it will not produce bilirubin.24Protecting the specimen from light will minimize the conversion of RBCs to oxyhemoglobin. Alternatively, spectrophotometry can differentiate the oxyhemoglobin of traumatic tap from the bilirubin of SAH. Visual inspection, however, is still used in most institutions.
What causes headaches in the ED?
Headache caused by a subarachnoid hematoma (SAH) from a ruptured aneurysm is one of the most deadly, with a median case-fatality of 27–44%.2Fortunately, it is also rare, comprising only 1% of all headaches presenting to the ED.3On initial presentation, the one-year mortality of untreatedSAH is up to 65%.4With appropriate diagnosis and treatment, mortality can be reduced to 18%.5
What is the post test probability of disease for a CTA without aneurysm?
Based on best available literature, a CTA without findings of aneurysm when coupled with a negative non-contrast head CT has a post-test probability of disease of < 1%.31This percentage is important because it falls below most clinicians’ test threshold, which is the probability of disease below which no further investigation is required. However there is one confounding factor in this suggested algorithm (Figure 2). The sensitivity of CTA is 92.3% for aneurysms < 4mm,32and in contrast to pathologies where the size of the lesion correlates with the severity of disease (i.e., pulmonary embolus), a small, ruptured cerebral aneurysm can still lead to significant morbidity and mortality.
How sensitive is a CT scan for a thunderclap headache?
While the results carry many of the limitations of a meta-analysis, a conservative statistical analysis showed that a non-contrast CT completed within six hours of headache onset had a sensitivity of 98.7% with confidence intervals 97.1%–99.4%. The authors took into consideration the following criteria: patient must have a hematocrit > 30% and an isolated thunderclap headache without seizure, syncope, or neck pain; and the CT image must be third generation or newer, of high quality, read by an attending-level radiologist, and evaluated with the indication for imaging being thunderclap headache or concern for SAH. If these criteria are met, many consider a negative head CT within six hours to be a “rule-out” study given the sensitivity and confidence intervals.
What percentage of strokes are caused by intracerebral hemorrhage?
An intracerebral hemorrhage (ICH) account for only 15% of all strokes but it is one of the most disabling forms of stroke (Counsell et al 1995; Qureshi et al 2005). Greater than one third of patients with intracerebral hemorrhage (ICH) will not survive and only twenty percent of patients will regain functional independence (Counsell et al 1995). This high rate of morbidity and mortality has prompted investigations for new medical and surgical therapies for intracerebral hemorrhage.
What is secondary ICH?
Secondary ICH is due to underlying vascular malformation, hemorrhagic conversion of an ischemic stroke, coagulopathy, intracranial tumor, etc. Arteriovenous malformations and cavernous malformations account for majority of underlying vascular malformations (Sutherland and Auer 2006). An AVM (Figure 2) is usually a singular lesion composed of an abnormal direct connection between distal arteries and veins. AVMs account for only 2% of all ICH but are associated with an 18% annual rebleed risk (Al-Shahi and Warlow 2001). Cavernous malformations are composed of sinusoidal vessels and are typically located in within the supratentorial white matter. The annual risk of recurrent hemorrhage is only 4.5% (Konziolka and Bernstein 1987). Intracranial aneurysms usually present with subarachnoid hemorrhage but anterior communicating artery and middle cerebral artery may also have a parenchymal hemorrhagic component near the interhemispheric fissure and perisylvian region respectively (Wintermark and Chaalaron 2003). Embolic ischemic strokes can often demonstrate hemorrhagic conversion without significant mass effect (Ott and Zamani 1986). Sinus thrombosis should be suspected in patients with signs and symptoms suggestive of increased intracranial pressure and radiographic evidence of superficial cortical or bilateral symmetric hemorrhages (Canhoe and Ferro 2005). An underlying cogenial or acquired coagulopathy causing platelet or coagulation cascade dysfunction can result in ICH. Cogenial disorders account for Hemophilia A, Hemophilia B, and other rare diseases. Acquired coagulopathy may be attributed to longstanding liver disease, renal disease, malignancy, or medication. Particular attention has been directed towards oral anticoagulant (OAT) associated hemorrhage due to greater risk for hematoma expansion as well as increased 30 day morbidity and mortality rates (Flibotte et al 2004; Roquer et al 2005; Toyoda et al 2005; Steiner and Rosand 2006). Metastatic tumors account for less than ten percent of ICH located near the grey white junction with significant mass effect. The primary malignancy is usually melanoma, choriocarninoma, renal carcinoma, or thyroid carcinoma (Kondziolka and Berstein 1987).
What is primary ICH?
Primary ICH develops in the absence of any underlying vascular malformation or coagulopathy. Primary intracerebral hemorrhage is more common than secondary intracerebral hemorrhage. Hypertensive arteriosclerosis and cerebral amyloid angiopathy (CAA) are responsible for 80% of primary hemorrhages (Sutherland and Auer 2006). At times it may be difficult to identify the underlying etiology because poorly controlled hypertension is often identified in most ICH patients. Patients with CAA-related ICH are more likely to be older and the volume of hemorrhage is usually > 30 cc (Ritter et al 2005). Hypertension related ICH is frequently seen in younger patients, involving the basal ganglia, and the volume of blood is usually < 30 cc (Lang et al 2001). However these characteristics are nonspecific and histopathological studies are needed to confirm a definitive diagnosis of CAA or hypertension related ICH. Hypertension causes high pressure within the Circle of Willis resulting in smooth cell proliferation followed by smooth muscle cell death. This may explain why hypertension related ICH are frequently located deep within the basal ganglia, thalamus (Figure 1), cerebellum, pons and rarely the neocortex (Campbell and Toach 1981; Sutherland and Auer 2006). In contrast, preferential amyloid deposition within leptomeningeal and intraparenchymal cortical vessels may explain the reason for large superficial lobar hemorrhages with amyloid angiopathy (Auer and Sutherland 2005). It is important to identify those afflicted with cerebral amyloid angiopathy because of the high risk of recurrent lobar hemorrhage and predisposition for symptomatic hemorrhage with anticoagulants and thrombolytics (Rosand and Greenberg 2000).
What to do for fever after ICH?
Fever after ICH is common and should be treated aggressively because it is independently associated with a poor outcome (Schwarcz et al 2001). Sustained fever in excess of 38.3 °C (101.0 °F) should be treated with acetaminophen and cooling blankets. Patients should be physically examined and should undergo laboratory testing or imaging to determine the source of infection. Fever of neurologic origin is diagnosis of exclusion and may be seen when blood extends into the subarachnoid or intraventricular (Commichau and Scarmeas 2003). Intracerebral hemorrhage patients with persistent fever that is refractory to acetaminophen and without infectious cause may require cooling devices to become normothermic. Adhesive surface-cooling systems and endovascular heat-exchange catheters are better at maintaining normothermia than conventional treatment. However, it is still unclear whether maintaining normothermia will improve clinical outcome (Dringer 2004).
How long does it take for a seizure to occur after ICH?
The 30-day risk of seizures after ICH is about 8%. Seizures most commonly occur at the onset of hemorrhage and may even be the presenting symptom. Lobar location is an independent predictor of early seizures (Passero et al 2003). Although, no randomised trial has addressed the efficacy of prophylactic antiepileptic in ICH patients, the Stroke Council of the American Heart Association suggest prophylactic antiepileptic treatment may be considered for 1 month in patients with intracerebral hemorrhage and discontinued if no seizures are noted (Broderick et al 1999; Temkin 2001). Acute management of seizures entail administering intravenous lorazepam (0.05–0.10 mg/kg) followed by an intravenous loading dose of phenytoin or fosphenytoin (15–20 mg/kg), valproic acid (15–45 mg/kg), or phenobarbital (15–20 mg/kg).
What are the symptoms of ICH?
The classic presentation of ICH is sudden onset of a focal neurological deficit that progresses over minutes to hours with accompanying headache, nausea, vomiting, decreased consciousness, and elevated blood pressure. Rarely patients present with symptoms upon awakening from sleep. Neurologic deficits are related to the site of parenchymal hemorrhage. Thus, ataxia is the initial deficit noted in cerebellar hemorrhage, whereas weakness may be the initial symptom with a basal ganglia hemorrhage. Early progression of neurologic deficits and decreased level of consciousness can be expected in 50% of patients with ICH. The progression of neurological deficits in many patients with an ICH is frequently due to ongoing bleeding and enlargement of the hematoma during the first few hours (Kazui et al 1996; Brott et al 1997; Fujii et al 1998). Compared with patients with ischemic stroke, head ache and vomiting at onset of symptoms is observed three times more often in patients with ICH (Gorlick et al 1986; Rathore et al 2002). Despite the differences in clinical presentation between hemorrhagic and ischemic strokes, brain imaging is required to definitively diagnose intracerebral hemorrhage.
Which stroke has the highest mortality rate?
Currently, intracerebral hemorrhage (ICH) has the highest mortality rate of all stroke subtypes (Counsell et al 1995; Qureshi et al 2005). Hematoma growth is a principal cause of early neurological deterioration. Prospective and retrospective studies indicate that up to 38% hematoma expansion is noted within three hours of ICH onset and that hematoma volume is an important predictor of 30-day mortality (Brott et al 1997; Qureshi et al 2005). This article will review current standard of care measures for ICH patients and new research directed at early hemostatic therapy and minimally invasive surgery.
What is primary IVH?
Primary IVH is confined to the ventricular system, arising from an intraventricular source or a lesion contiguous to the ventricles. Examples include intraventricular trauma, aneurysm, vascular malformation, and tumor, usually involving the choroid plexus. Approximately 70% of IVHs are secondary; secondary IVHs may occur as an extension of an intraparenchymal hemorrhage or SAH into the ventricular system. Risk factors for IVH include older age, higher baseline ICH volume, mean arterial pressure values greater than 120 mm Hg, and location of the primary ICH [7]. Deep, subcortical structures tend to be most at risk for IVH; frequent locations include the putamen (35%–50%), lobes (30%), thalamus (10%–15%), pons (5%–12%), caudate (7%), and cerebellum (5%) [8]. Whereas some authors have focused on the volume of the original ICH as the predictor of poor outcome, others have used sophisticated volumetrics to define a threshold volume of IVH (20 mL) as particularly ominous [9]. Hallevi et al. [10••] correlated larger ICH volume with the presence of IVH, as well as location near the ventricular system, which likely leads to early intraventricular rupture.
When was intraventricular RT-PA first used?
The first use of intraventricular rt-PA in humans appeared in the literature in 1991 [61]. Beneficial effects on clot resolution, ICP, ventricular size, and mortality have been seen in observational studies with both rt-PA and urokinase, although urokinase is not currently available in the United States [6, 62–64]. At present, intraventricular rt-PA for severe IVH is not approved by the US Food and Drug Administration, and its use in this capacity is considered off-label.
Which stroke has the highest morbidity and mortality?
Brain hemorrhage has the highest morbidity and mortality of any stroke subtype. Respectively, intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) account for about 15% and 5% of the 750,000 strokes occurring yearly in the United States, totaling more than 45,000 patients per year [1–3]. About 45% of spontaneous ICHs and 25% of aneurysmal SAHs extend into the ventricles [1, 4, 5]. For patients with both ICH and intraventricular hemorrhage (IVH), the expected mortality is 50% to 80% [6, 7]. Patients with IVH are twice as likely to have poor outcomes (a modified Rankin scale [mRS] score of 4–6 at hospital discharge) and nearly three times more likely to die than their cohorts without IVH [8].
Does IVH cause coma?
Often it is assumed that coma and death associated with IVH result from an acute rise in ICP that injures the reticular activating system or compromises cerebral perfusion. Nevertheless, no correlation between ICP and outcome has been reported for IVH. Diringer et al. [43] found that hydrocephalus increased mortality and produced higher intubation rates in patients with IVH. A Japanese study of 35 patients with IVH and ICH found that IVH severity influenced the occurrence of acute hydrocephalus and initial level of consciousness, which was significantly associated with prognosis. The authors concluded that priority treatment of the IVH should be given to patients with a Graeb score ≥ 6 [12]. Adams and Diringer [44] reported that control of initial ICP with an external ventricular drain (EVD) had little impact on ventricular size or level of consciousness in their cohort of 22 patients. The authors speculated that parenchymal and/or IVH clot volume may have overshadowed the influence of hydrocephalus. Mortality in this series was 73%. Coplin et al. [6] reported on a cohort of 40 patients with spontaneous IVH who received EVDs. The mean initial ICP was 15.6 mm Hg, and only six patients (15%) had ICP elevation (ICP > 20 mm Hg) at the time of EVD placement. We analyzed every-4-hour ICP readings from 11 IVH patients who required an EVD and found that initial ICP at the time of EVD placement was uncommonly elevated (1 of 11 patients) despite acute obstructive hydrocephalus [45]. ICP elevation greater than 20 mm Hg occurred in only 14% of observations during EVD use, and the EVD was equally effective in controlling ICP in patients treated with intraventricular urokinase or placebo. In this study, only 2 of 141 intraventricular injections of study agent, followed by EVD closure for 1 h, were not tolerated and required reopening of the EVD. All these results support the concept that acute obstructive hydrocephalus remains a potentially lethal complication of IVH but that ICP may not be the only factor determining the harmful effect of IVH. The presence of a ventricular drain, which is kept open to drain at a clinically rational height, may explain the low incidence of ICP greater than 20 mm Hg. However, in this study, patients were required to have an intraparenchymal hematoma volume less than 30 mL and no significant coagulopathy or aneurysmal or arteriovenous malformation–related ICH. These latter populations frequently experience intracranial hypertension; therefore, the significance of ICP across the full spectrum of IVH patients is not known.
Is IVC placement a serious problem?
Generally, IVC placement is well tolerated, with a relatively low incidence of serious complications. The most frequent complications are hemorrhage, (intraparenchymal, intraventricular, or subdural), catheter-related infection, and obstruction requiring reinsertion. Catheter-related hemorrhages reportedly occur in 1% to 33% of patients [2, 7, 29, 30, 47–49]. This wide range likely is a result of the lack of a standard definition or imaging technique. We propose defining catheter tract hemorrhages by size (small, < 5 mm; large > 5 mm) and whether they are symptomatic or asymptomatic. IVC-related infection has been reported in 1–12% of patients [12, 36–39, 51]. Currently, antibiotics are administered prophylactically either perioperatively or continuously, although previous investigations have not demonstrated an advantage of one strategy over the other. Our practice is to give a single periprocedural dose of oxacillin. Malpositioning of IVCs is not infrequent but clearly depends on definition. In a study of 169 EVD and 43 shunt procedures at the Mayo Clinic, 26 ventriculostomy catheters (12.3%) were malpositioned as determined by postprocedural imaging [49]. Most misplacements involved an intraparenchymal distal catheter tip, whereas five were in other extraventricular spaces. The need for IVC replacement is common after IVH, usually because of blood clots occluding the catheter. In a prospective case-control study of 59 patients with IVH requiring an IVC, the catheter had to be replaced in 59% of patients who did not receive intraventricular thrombolytic therapy and 32% of those who did [51].
Is hematoma growth a determinant of mortality?
Hematoma growth is an independent determinant of both mortality and functional outcome after ICH [42]. In a secondary analysis of data from the multicenter, randomized, placebo-controlled trial of rFVIIa effectiveness in spontaneous ICH, 170 of 374 patients (45%) had IVH at baseline and 12% (44 of 374) had a greater than 2 mL increase in IVH volume between baseline and 24-hour CT scan [7]. Limiting intraventricular hematoma growth may be an important therapeutic target.
Does warfarin cause ICH?
Anticoagulation with warfarin raises the risk of intracranial hemorrhage 7- to 10-fold [36]. Treating to an international normalized ratio of 1.4 may reduce the risk of progressive bleeding and clinical deterioration. Time to treatment with reversal agents is an important determinant of 24-hour anticoagulation reversal. It is recommended that a fresh frozen plasma infusion followed by oral vitamin K be given without delay in the emergency department to manage warfarin-related ICH [37]. Alternately, prothrombin complex concentrate and intravenous vitamin K may be given if volume overload is a concern. Additionally, there is evidence that reduced platelet activity is associated with larger IVH volume, which may relate to aspirin use [38]. In a prospective cohort of 73 patients with spontaneous ICH, 36 of whom had IVH, higher Graeb scores (IVH severity) were associated with less platelet activity (VerifyNow-Aspirin assay; Accumetrics, San Diego, CA) after correcting for ICH volume and location. At present, there is no robust evidence for transfusing platelets in patients taking platelet inhibitors such as aspirin and clopidogrel.
How to prevent rebleeding of aneurysm?
Prevention of rebleeding may be achieved by treating acute hypertension, repairing the aneurysm as soon as possible, and administering antifibrinolytic drugs. The median time to rebleeding was 180 minutes in 48 patients (16%) of 293 patients. 31 Aneurysms would have to be repaired emergently if this is true. That has not been done in a RCT. Guidelines suggest repairing the aneurysm as early as feasible. Therefore, reducing blood pressure should reduce the transmural pressure difference across the aneurysm wall and thereby reduce rebleeding. Data, however, do not confirm this. Emergently reducing systolic blood pressure to <140 mm Hg in a consecutive series of 309 patients with aSAH was associated with a higher risk of rebleeding of 14% compared with 6% for those with systolic blood pressure >140 mm Hg. 32
What causes cerebral ischemia after aSAH?
Increased intracranial pressure causing global cerebral ischemia, as well as blood-brain barrier breakdown, global cerebral edema , and subarachnoid blood toxicity theoretically contribute to early brain injury within 72 hours of aSAH (Figure V in the Data Supplement ). The clinical correlate would be the neurological grade of the patient. 38 Edema can be inferred from CT findings of loss of gray-white differentiation and obliteration of sulci. 39 High-grade global cerebral edema after SAH was identified in 48 of 164 (29%) patients and was an independent predictor of DCI and unfavorable outcome although this depended on which variables were adjusted for. 40 Glyburide is a promising drug undergoing RCT in ischemic stroke for reducing brain edema and should be considered for SAH RCT. 41
How long after SAH can you take antifibrinolytics?
Administering antifibrinolytic drugs before aneurysm repair, for a maximum of 72 hours after SAH has been advocated to reduce rebleeding. An open-label study included 505 patients randomized to receive tranexamic acid for up to 72 hours or not found no significant improvement in 6-month outcome. There is an RCT studying short-term tranexamic acid use. 33
Can endovascular repair be performed on an aneurysm?
Endovascular repair now is performed for most ruptured aneurysms, but prac titioners are using an increasing variety of coils, stents, and devices that have never been tested in RCT and that are applied in cases that would not have been included in the original study (Figures III and IV in the Data Supplement ). 34 An RCT to compare neurosurgical clipping and endovascular repair of aneurysms that would not have been included in ISAT (International Subarachnoid Aneurysm Trial) is ongoing.
Can a CT scan detect sudden headaches?
7 Computed tomography (CT) is the first diagnostic test, but this results in many patients undergoing CT to detect one SAH (Figures I and II in the Data Supplement ). An SAH rule to select patients with sudden headache who were likely to have SAH had 100% sensitivity and 14% specificity for detecting SAH in a prospective cohort of 1153 neurologically intact patients with sudden headache (Table III in the Data Supplement ). 7 The authors recommended more prospective studies to validate this decision tool.
Is nimodipine effective for SAH?
Outcome has improved over time although the only treatments shown to be effective in adequate, well-controlled clinical trials are nimodipine and repair of the ruptured aneurysm by coiling rather than clipping. All other management is based on less evidence, leading to variation in guidelines and management (Tables I and II in the Data Supplement ). 4–6 The aim of this article is not to update guidelines but to review the diagnosis and treatment of SAH focusing on recent advances, the basis for these advances, to identify gaps in knowledge, and to suggest areas for future studies.
Is rebleeding before aneurysm repair preventable?
Rebleeding before aneurysm repair may be the most preventable serious complication of aSAH. 28 Meta-analysis of 14 studies including 5693 patients reported rebleeding in 7% to 26% of patients (mean 13%). 29 Factors associated with rebleeding were temporal proximity to the first hemorrhage, higher blood pressure, worse neurological grade, intraventricular or intracerebral hemorrhage, and larger aneurysm. A score to predict rebleeding found that rebleeding was increased in patients with preexisting hypertension, posterior circulation aneurysm, larger aneurysm, intracerebral hemorrhage, and acute hydrocephalus. 30

Description of The Problem
- Broadly classified as subtypes of stroke, subarachnoid and intracerebral hemorrhage are commonly seen in the critical care unit. Intracerebral Hemorrhage (ICH) Defined as a hemorrhage into the brain parenchyma; usually arises in the region of the small arteries that supply the basal …
Emergency Management
- Emergent management of both ICH and SAH centers around rapid diagnosis, blood pressure management, and referral to a center with the appropriate neurosurgical and neurocritical care capabilities. Patients may be obtunded to the point that they require intubation for airway protection. If necessary, this should be accomplished with good hemodynamic control. Blood pr…
Diagnosis
- 1. CT Head (non-contrast): the study of choice to diagnose the bleed, its size, and its location. CT angiography can be added at medical centers with this capability in order to look for underlying vascular lesions. For SAH: approximately 90% of hemorrhages are visible on CT within 24 hours of the ictus; the thickness of the clot and the presence of intraventricular hemorrhage predict the ri…
Epidemiology
- ICH: occurs in 12-30 per 100,000 people each year in the United States, accounts for 10% of all strokes, with a mortality rate of 30-50%. SAH accounts for 5% of all strokes. It affects 30,000 individuals per year in the United States. Approximately 10% of those patients die before receiving medical attention, and another 20% die after hospitalization.
Prognosis
- SAH: 10% of patients die before reaching the hospital, another 20% die after hospitalization, and a majority of survivors have some neurocognitive impairments. There are several predictors for poor outcome following SAH, including Glasgow Coma Scale score on presentation, Hunt-Hess grade, aneurysm size, age, rebleeding, vasospasm, hydrocephalus, hyponatremia and seizures. I…
What's The Evidence?
- Gebel, JM. “Intracerebral hemorrhage”. Neurol Clin. vol. 18. 2000. pp. 419-38. Broderick, J, Connolly, S. “Guidelines for management of spontaneous ICH in adult”. Stroke. vol. 38. 2010. pp. 2001-23. Hemphill, JC. “Reliable grading scale for ICH, ICH score”. Stroke. vol. 32. 2001. pp. 891-7. Mast, H. “Risk of spontaneous hemorrhage after diagnosis of AVM”. Lancet. vol. 350. 1997. pp. …