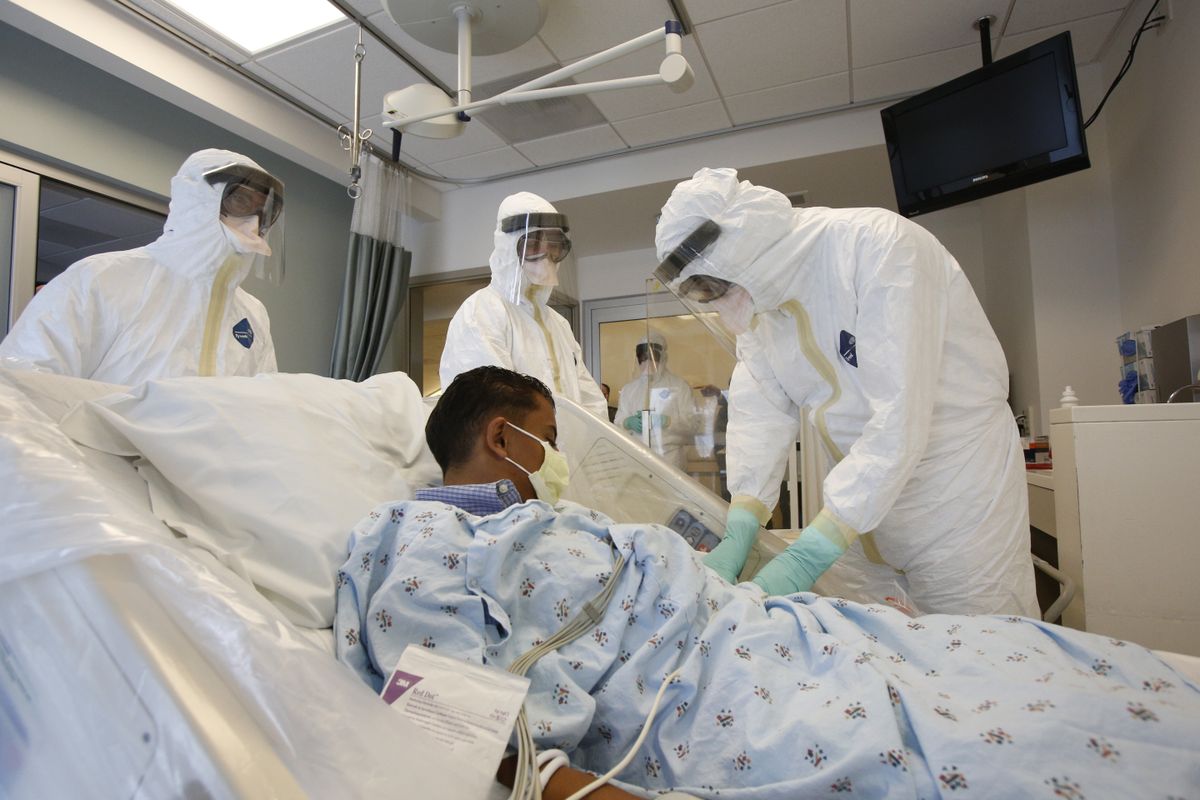
Therapy
Treatment options for patients infected with Ebola virus are limited. Supportive therapy is centered on fluid resuscitation, electrolyte imbalance correction, treating complicating infections, and preventing complications of shock. Experimental therapies (ZMapp, brincidofovir, TKM-Ebola, and favipiravir) have been used during this current outbreak.
Self-care
Does Ebola really make people bleed from their eyes? Yes. Bleeding from orifices is one of the more unusual and memorable symptoms of viral hemorrhagic fevers like Ebola. In later stages of the disease, some people bleed from the eyes, nose, ears, mouth, and rectum. They may also bleed from puncture sites if they've had an IV.
Nutrition
Tips to help you get the most from a visit to your healthcare provider:
- Know the reason for your visit and what you want to happen.
- Before your visit, write down questions you want answered.
- Bring someone with you to help you ask questions and remember what your provider tells you.
- At the visit, write down the name of a new diagnosis, and any new medicines, treatments, or tests. ...
What are the treatment options for Ebola?
What They’re Not Telling You: 4 Natural Ways to Fight Ebola
- Genistein. An organic compound found primarily in soy products, genistein has shown much promise when combined with fellow kinase inhibitor tyrphostin AG1478.
- Garcinia Kola. A tree found throughout Western Africa, Garcinia kola has been found to “inhibit the Ebola virus in cell culture at non-toxic concentrations.”
- Vitamin C. ...
- Estradiol. ...
Does Ebola really make people bleed from their eyes?
What is the best medicine for Ebola virus?
How can you treat Ebola?

How is Ebola medically treated?
Providing fluids and electrolytes (body salts) orally or through infusion into the vein (intravenously). Using medication to support blood pressure, reduce vomiting and diarrhea, and to manage fever and pain.
Who found the medicine for Ebola?
BCX4430 is a broad-spectrum nucleoside analog antiviral drug developed by BioCryst Pharmaceuticals. A phase one trial started in December 2014. The drug was effective in Ebola-infected monkeys.
Is there a cure or vaccine for Ebola?
There's no cure for Ebola, though researchers are working on it. There are two drug treatments which have been approved for treating Ebola.
Is there a vaccine for Ebola?
Currently there are no licensed vaccines to prevent Ebola virus disease. However, multiple investigational Ebola vaccines have been tested in numerous clinical trials around the world. NIAID has supported the development of various candidates, including the rVSV-ZEBOV vaccine developed by Merck.
What is the cause of Ebola?
Ebola is caused by viruses in the Ebolavirus and Filoviridae family. Ebola is considered a zoonosis, meaning that the virus is present in animals and is transmitted to humans.
How many people were involved in the Ebola trial?
published preliminary results of a vaccine trial funded and organized by the WHO; the Ebola ca Suffit vaccine had 100 percent efficacy in the trial, which took place in Guinea and involved 4,000 people. The full results of this trial were published in Lancet. Trusted Source. in February 2017.
How does Ebola transmission occur in humans?
When an Ebola infection occurs in humans, the virus can be spread in several ways to others. Above is a list of ways Ebola can and cannot be transmitted.
How long does it take for Ebola to show symptoms?
Symptoms of Ebola. The time interval from infection with Ebola to the onset of symptoms is 2-21 days, although 8-10 days is most common. Signs and symptoms include: fever. headache. joint and muscle aches. weakness. diarrhea. vomiting.
What animals have Ebola?
In Africa, people have developed Ebola after handling infected animals found ill or dead, including chimpanzees, gorillas, fruit bats, monkeys, forest antelope, and porcupines. Person-to-person transmission occurs after someone infected with Ebolavirus becomes symptomatic.
Where did Ebola first appear?
The first cases of Ebola were reported simultaneously in 1976 in Yambuku, near the Ebola River in Zaire ( now the Democratic Republic of the Congo) and in Nzara, Sudan .
Where has Ebola been found?
Since then, eruptions or asymptomatic cases of Ebola in humans and animals have surfaced intermittently in the following locations due to outbreaks, laboratory contamination, and accidents: The Democratic Republic of the Congo (DRC) Sudan (South Sudan) Senegal. United Kingdom. United States (U.S.) Philippines. Italy.
What animals can you touch to get rid of Ebola?
Touching the body of someone who has died from an Ebola virus infection. Contact with certain animals such as bats, monkeys and chimps. Eating bushmeat (the meat of wild animals such as bats, antelope and monkeys). Care providers need to take extra care to avoid getting or spreading Ebola.
What are the symptoms of Ebola?
Ebola symptoms include fever, pain and bleeding. Treatment improves the chance of survival. Overview. Symptoms and Causes. Diagnosis and Tests. Management and Treatment. Prevention. Outlook / Prognosis. Living With.
What is the cause of Ebola?
Ebola is caused by a virus from the group of viral hemorrhagic fever (VHF) viruses (Marburg virus is another). Infection occurs by direct contact with infected body fluids - blood, diarrhea, saliva (“spit”), mucus (“snot”), urine (“pee”), vomit (“puke”), breast milk or semen from an infected person or animal (bat, monkey or ape).
Where did the Ebola virus occur?
It has occurred in Central and West Africa but can be carried and spread by travelers from this region. The largest outbreaks occurred in 2014-2016 mostly in Liberia, Guinea and Sierra Leone. On average, 50% of people who get Ebola virus disease die.
Can you get Ebola from a bat?
After direct contact you then touch your eyes, nose, mouth or an area of broken skin. The infected body fluids can be on a person or object. You can also get Ebola virus disease from an infected bat, monkey or ape. It is not spread through the air.
Does Inmazeb help with Ebola?
Patients who take Inmazeb have a higher chance of survival. Healthcare providers treat Ebola virus symptoms to improve the chance of survival. Treatments include giving: IV or fluids and electrolytes (body salts). Medicine to control fever, diarrhea and vomiting. Oxygen.
What is the name of the Ebola trial?
The trial, known as PALM (short for “Pamoja Tulinde Maisha” a Swahili phrase which roughly translates to “together save lives”) ...
What is the name of the trial that was conducted by the World Health Organization?
The trial, known as PALM (short for “Pamoja Tulinde Maisha” a Swahili phrase which roughly translates to “together save lives”) was conducted by a research consortium overseen by the World Health Organization that included nongovernment organizations and the DRC Ministry of Health.
What is Ebanga used for?
Food and Drug Administration approved Ebanga (Ansuvimab-zykl), a human monoclonal antibody, for the treatment for Zaire ebolavirus (Ebolavirus) infection in adults and children. Ebanga blocks binding of the virus to the cell receptor, preventing its entry into the cell.
What is the Ebanga drug designation?
Ebanga was granted an Orphan Drug designation, which provides incentives to assist and encourage drug development for rare diseases. Additionally, the agency granted Ebanga a Breakthrough Therapy designation.
What are the symptoms of Ebanga?
The most common symptoms experienced while receiving Ebanga include: fever, tachycardia (fast heart rate), diarrhea, vomiting, hypotension (low blood pressure), tachypnea (fast breathing) and chills; however, these are also common symptoms of Ebolavirus infection. Hypersensitivity, including infusion-related events, can occur in patients taking Ebanga, and treatment should be discontinued in the event of a hypersensitivity reaction.
When was Ebanga evaluated?
During an Ebola outbreak in the Democratic Republic of the Congo (DRC) in 2018-2019, Ebanga was evaluated in a clinical trial (the PALM trial). The PALM trial was led by the U.S. National Institutes of Health and the DRC’s Institut National de Recherche Biomédicale with contributions from several other international organizations and agencies.
Diagnosis
Ebola virus and Marburg virus are difficult to diagnose because early signs and symptoms resemble those of other diseases, such as typhoid and malaria. If doctors suspect that you have Ebola virus or Marburg virus, they use blood tests to quickly identify the virus, including:
Treatment
The U.S. Food and Drug Administration (FDA) has approved a medication that's a combination of three monoclonal antibodies (Inmazeb) and a single monoclonal antibody medication (Ebanga) to treat Ebola virus disease caused by a specific type of Ebola virus.
Preparing for your appointment
The possibility of getting Ebola virus or Marburg virus is extremely low unless you've had direct contact with the body fluids of a person or an animal infected with one of the viruses.
What is the target of Inmazeb?
Inmazeb targets the glycoprotein that is on the surface of Ebola virus. Glycoprotein attaches to the cell receptor and fuses the viral and host cell membranes allowing the virus to enter the cell.
What is an orphan drug?
The Orphan Drug designation provides incentives to assist and encourage drug development for rare diseases. Additionally, the agency granted Inmazeb a Breakthrough Therapy designation for the treatment of Zaire ebolavirus infection. The FDA is granting the approval to Regeneron Pharmaceuticals. The FDA approved Ervebo, the first vaccine for ...
What are the symptoms of Inmazeb?
The most common symptoms experienced while receiving Inmazeb included: fever, chills, tachycardia (fast heart rate), tachypnea (fast breathing), and vomiting; however, these are also common symptoms of Ebola virus infection. Patients who receive Inmazeb should avoid the concurrent administration of a live vaccine due to ...
When will Inmazeb be released?
For Immediate Release: October 14, 2020. Today, the U.S. Food and Drug Administration approved Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn), a mixture of three monoclonal antibodies, as the first FDA-approved treatment for Zaire ebolavirus (Ebola virus) infection in adult and pediatric patients. “Today’s action demonstrates the FDA’s ...
Can Inmazeb be discontinued?
Hypersensitivity, including infusion-related events, can occur in patients taking Inmazeb, and treatment should be discontinued in the event of a hypersensitivity reaction. Inmazeb received an Orphan Drug designation for the treatment of Ebola virus infection.
Is Inmazeb a clinical trial?
Inmazeb was evaluated in 382 adult and pediatric patients with confirmed Zaire ebolavirus infection in one clinical trial (the PALM trial) and as part of an expanded access program conducted in the Democratic Republic of the Congo (DRC) during an Ebola virus outbreak in 2018-2019. The PALM trial was led by the U.S. National Institutes of Health and the DRC’s Institut National de Recherche Biomédicale with contributions from several other international organizations and agencies.