Is there a drug treatment for COVID-19?
The U.S. Food and Drug Administration has approved one drug treatment for COVID-19 and has authorized others for emergency use during this public health emergency. In addition, many more therapies are being tested in clinical trials to evaluate whether they are safe and effective in combating COVID-19.Jan 27, 2022
Which drug is approved by FDA to treat COVID-19?
Veklury (Remdesivir) is an antiviral drug approved for use in adults and pediatric patients [12 years of age and older and weighing at least 40 kilograms (about 88 pounds)] for the treatment of COVID-19 requiring hospitalization.Mar 31, 2022
What antiviral drugs are available for treatment of COVID-19?
Remdesivir is the only drug that is approved by the Food and Drug Administration (FDA) for the treatment of COVID-19. Ritonavir-boosted nirmatrelvir (Paxlovid), molnupiravir, and certain anti-SARS-CoV-2 monoclonal antibodies (mAbs) have received Emergency Use Authorizations from the FDA for the treatment of COVID-19.Feb 24, 2022
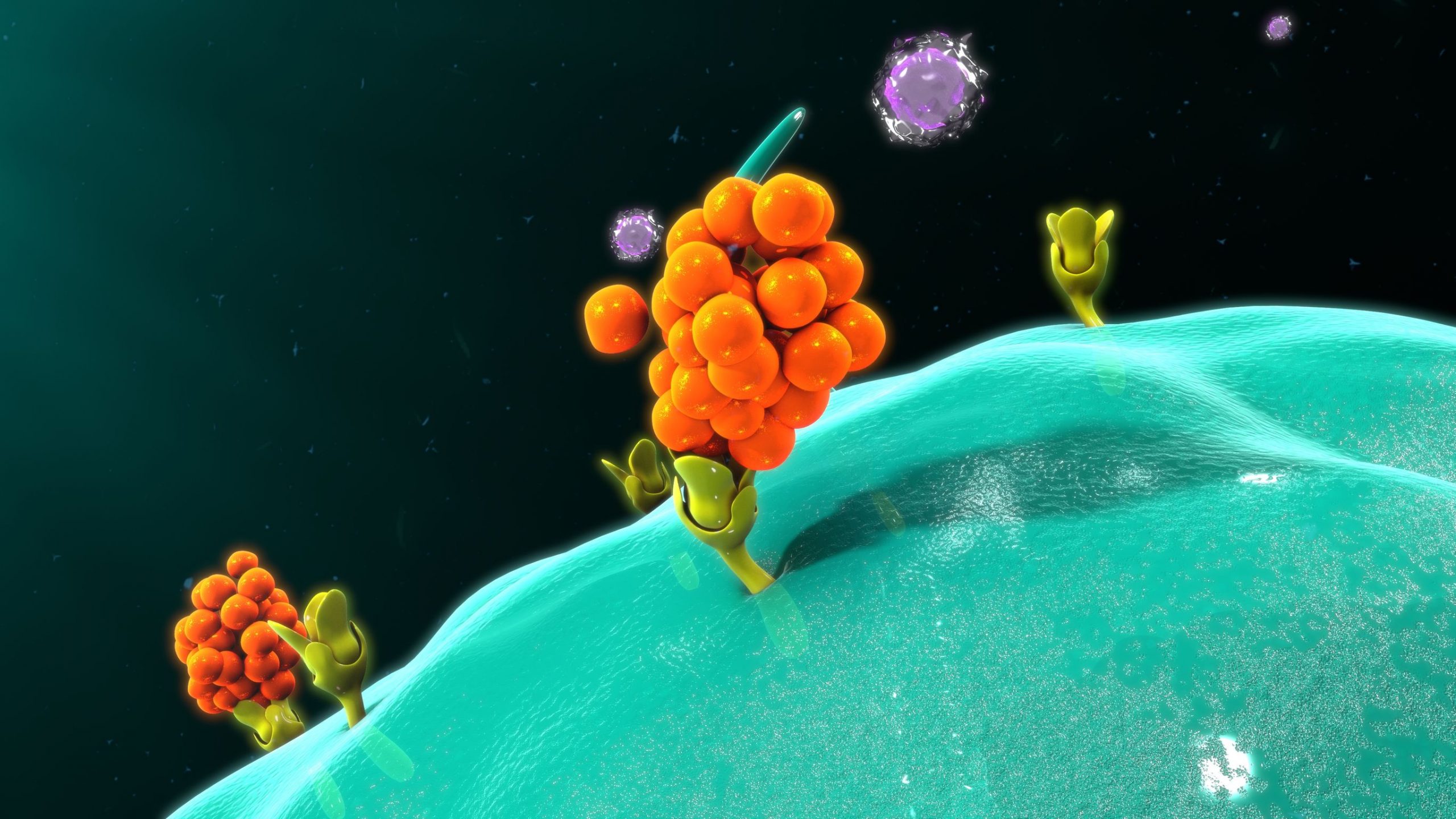
What is a monoclonal antibody for COVID-19?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells. Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
Is Remdesivir approved for treatment of COVID-19?
Remdesivir is approved by the Food and Drug Administration (FDA) for the treatment of COVID-19 in hospitalized adult and pediatric patients (aged ≥12 years and weighing ≥40 kg).
Is AstraZeneca approved by the FDA for COVID-19?
AstraZeneca is planning to seek full FDA approval of its COVID-19 vaccine by the end of the year, the company said July 29. More than 170 countries have authorized use of AstraZeneca's shot, but it hasn't been authorized for use in the U.S.Jul 29, 2021
What are the side effects of Remdesivir?
Remdesivir may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:• nausea• constipation• pain, bleeding, bruising of the skin, soreness, or swelling near the place where the medication was injected
Is the antiviral medication Paxlovid authorized for COVID-19?
On Dec 22, 2021, the US Food and Drug Administration (FDA) issued an emergency use authorisation for Pfizer's COVID-19 antiviral, Paxlovid.Jan 13, 2022
What is Remdesivir?
Remdesivir is in a class of medications called antivirals. It works by stopping the virus from spreading in the body.
What is a monoclonal antibody?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.