
Monoclonal Antibody Treatment Chart
| Outpatient Treatment | Hospital Treatment | Post Exposure & High Risk | Prevention and High Risk | |
| Sotrovimab | X | |||
| Tocilizumab | X | |||
| Tixagevimab and cilgavimab (Evusheld) | X | |||
| Bebtelovimab | X | X |
Can you get booster after infusion?
Aug 31, 2021 · Antibodies designed to attack COVID-19 have been developed, and in several studies have been shown to reduce the risk of progressing to severe COVID-19 and hospitalization when given early to people who test positive for COVID-19. This therapy is given as an infusion through an IV at one of the UNC Health infusion centers.
Does Medicare cover antibody infusions?
Jan 28, 2021 · The point of antibody therapy is to reduce the chance that you will ever develop very serious symptoms that require hospitalization. Do not wait until your symptoms start to get worse. To see if you qualify for this treatment, request an appointment today or call 800-TEMPLE-MED (800-836-7536).
When to give monoclonal antibody treatment?
Monoclonal antibody treatment is a medicine used to treat COVID-positive individuals who are symptomatic and have certain medical conditions that may result in a higher risk of hospitalization. Patients must be referred by a licensed health care provider to be considered for antibody therapy. Due to limited supplies of the specific antibody therapy effective against the …
How long does the antibody infusion take?
Jan 06, 2022 · “Monoclonal antibodies are laboratory-made proteins that mimic the body’s immune system to fight off COVID-19 infection,” Spivak says. These antibodies are given to people directly through an intravenous (IV) infusion. …
What is a monoclonal antibody for COVID-19?
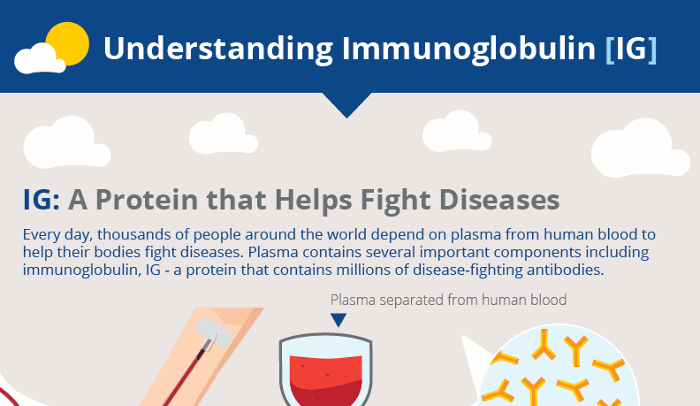
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells. Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Who could benefit from monoclonal antibody therapy to prevent COVID-19?
See full answerVaccines are the best way to protect against COVID-19. But some people with weakened immune systems do not produce enough antibodies after vaccination, and others are severely allergic to the vaccine. The FDA recently authorized Evusheld, a pre-exposure prophylaxis (PrEP) monoclonal antibody therapy developed by AstraZeneca, which should help prevent COVID-19 in these populations.To be eligible for Evusheld, individuals must be 12 years or older and have a moderately to severely weakened immune system, or have a history of severe adverse reactions to the COVID-19 vaccine or its components. In addition, the therapy cannot be given to someone with a current SARS-CoV-2 infection, or who has been recently exposed to someone who is infected. Evusheld is given as two consecutive shots, and evidence suggests it can help prevent symptomatic infection for at least six months.Apr 1, 2022
Are antibodies beneficial during the COVID-19 pandemic?
When reinfections or breakthrough infections happen, having antibodies plays an important role in helping prevent severe illness, hospitalization, and death. For many diseases, including COVID-19, antibodies are expected to decrease or “wane” over time.Nov 10, 2021
What is the first drug that was approved by the FDA to treat COVID-19?
Remdesivir is the first drug approved by the FDA for treatment of hospitalized COVID patients over the age of 12.Jan 25, 2022
Which drug is approved by FDA to treat COVID-19?
Veklury (Remdesivir) is an antiviral drug approved for use in adults and pediatric patients [12 years of age and older and weighing at least 40 kilograms (about 88 pounds)] for the treatment of COVID-19 requiring hospitalization.Mar 31, 2022
Who might benefit from dexamethasone if they have COVID-19?
Dexamethasone is a corticosteroid used in a wide range of conditions for its anti-inflammatory and immunosuppressant effects.It was tested in hospitalized patients with COVID-19 in the United Kingdom’s national clinical trial RECOVERY and was found to have benefits for critically ill patients.Oct 16, 2020
What do antibodies do to protect against COVID-19?
Antibodies are specialized proteins that are part of your immune system. They help protect against viruses, bacteria and other foreign substances. In the case of COVID-19, after you're infected with the SARS-CoV-2 virus, your immune system recognizes the virus as a foreign substance and forms antibodies against it.Nov 10, 2021
Who are some groups at higher risk for serious illness from COVID-19?
Some people may be at higher risk of severe illness. This includes older adults (65 years and older) and people of any age with serious underlying medical conditions. By using strategies that help prevent the spread of COVID-19 in the workplace, you will help protect all employees, including those at higher risk.
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
How long do COVID-19 antibodies last?
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.Jan 31, 2022
Can you get COVID-19 if you already had it and have antibodies?
It is important to remember that some people with antibodies to SARS-CoV-2 may become infected after vaccination (vaccine breakthrough infection) or after recovering from a past infection (reinfected).Nov 10, 2021
NOTE: Monoclonal antibody therapy doses containing the combination of casirivimab and imdevimab are free of charge
The U.S. government signed an agreement with Regeneron, the maker of casirivimab and imdevimab, so patients that need it would not be charged. Some patients, depending on their insurance coverage, may have to pay a fee to their healthcare provider for administering the dose.
What COVID-19 treatment is there for people outside the hospital?
If you are diagnosed with COVID-19 but aren’t sick enough to be hospitalized, you may think there isn’t much you can do. It is important to:
What are monoclonal antibodies?
Antibodies are naturally produced by your body to fight off infections. When your body is introduced to a new virus such as COVID-19, it does not have the antibodies to fight it off. That is where monoclonal antibodies come in. Monoclonal antibodies are created in a laboratory. They can target a particular virus or infection such as COVID-19.
How does monoclonal antibody infusion therapy work?
Monoclonal antibodies are given by IV to people diagnosed with COVID-19. This therapy uses COVID-19 antibodies to help a person’s body fight off the infection. Research suggests these antibodies lower the amount of virus — the “viral load” — in a person’s body. People with lower viral loads have more mild symptoms.
Who should get antibody infusion therapy?
Monoclonal antibodies are used for people with a positive COVID-19 test and symptoms for 10 days or less. The therapy for COVID-19 works best when given early in the COVID-19 illness. This is only recommended for those considered high risk for severe illness.
Who is at high risk for severe illness from COVID-19?
While anybody can get very sick or even die from COVID-19, those most at risk include:
What monoclonal antibody infusion therapies for COVID-19 are available?
The Food and Drug Administration (FDA) has approved emergency use authorization for four antibody infusion therapies:
What to know about a syringe?
You should make sure your doctor knows if you: 1 Have any allergies 2 Are pregnant 3 Are breastfeeding 4 Plan to become pregnant or to breastfeed 5 Have any serious illnesses 6 Are taking any prescription or over-the-counter-medications, or if you use vitamins or herbal products
What is an EUA?
What is emergency use authorization (EUA)? Regeneron and Eli Lilly antibody treatments are available under an Emergency Use Authorization from the FDA. Based on limited clinical trials, the benefits of antibody infusions appear to outweigh the risks for people who are under the greatest threat from COVID-19.
What is the function of antibodies?
Antibodies are proteins that exist in our bodies as part of our immune system to recognize and defend against harmful viruses and bacteria. Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
Does infusion cause nausea?
Some people may experience infusion-related side effects, such as nausea and dizziness, that are short-lived and go away on their own. As with any medication, there is the potential for mild or more severe allergic reactions, which are uncommon.
Post COVID Recovery
Post COVID-19 Care UTMB Health’s Post COVID Recovery Clinic helps you resume life as you lived it before COVID-19.
Important Information: Read before Scheduling!
You will be called by UTMB before your scheduled appointment. We will call the number you provide when you schedule.
