What are the dangers of monoclonal antibodies?
Jan 06, 2022 · Monoclonal antibody therapy is a way of treating COVID-19 for people who have tested positive, have had mild symptoms for seven days or less, and are at high risk for developing more serious symptoms. The goal of this therapy is to help prevent hospitalizations, reduce viral loads, and lessen symptom severity.
How effective is the monoclonal treatment?
Monoclonal antibodies, or mAbs, are made in a laboratory to fight a particular infection (in this case, SARS-CoV-2) and are given to you directly in an infusion. So the mAb treatment may help if you are at high risk for serious symptoms or a hospital stay. The mAb treatment for COVID-19 is different from a COVID-19 vaccine.
Can monoclonal antibodies kill you?
Treatment with COVID-19 monoclonal antibodies is done through a one-time intravenous (IV) infusion. Another option for COVID-19 therapy is an antiviral called Remdesivir. Remdesivir is approved by the FDA and helps reduce the effects of COVID-19. Remdesivir is given by an intravenous (IV) infusion over three (3) consecutive days.
What do you know about monoclonal antibody therapy?
The FDA authorized the use of these monoclonal antibody therapies to treat mild-to-moderate COVID-19 in adults and pediatric patients when both of these apply: The patient has a positive COVID-19 test result. The patient is at high risk for progressing to severe COVID-19, hospitalization, or both.

What is a monoclonal antibody?
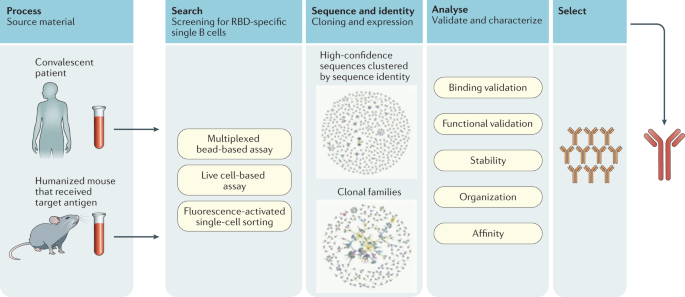
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
How do monoclonal antibodies work against COVID-19?
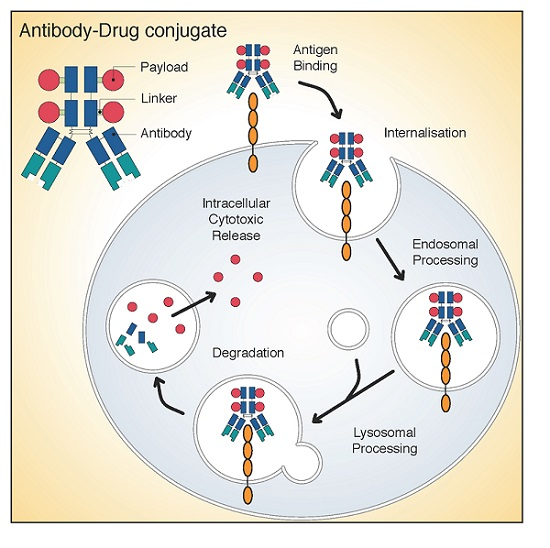
Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
What does monoclonal antibody treatment mean for COVID-19?
Monoclonal antibodies are manmade versions of the antibodies that our bodies naturally make to fight invaders, such as the SARS-CoV-2 virus.6 days ago
Which drug is approved by FDA to treat COVID-19?
Veklury (Remdesivir) is an antiviral drug approved for use in adults and pediatric patients [12 years of age and older and weighing at least 40 kilograms (about 88 pounds)] for the treatment of COVID-19 requiring hospitalization.Mar 31, 2022
How many types of COVID-19 vaccines are available in the US?
Three COVID-19 vaccines are authorized or approved for use in the United States to prevent COVID-19. Pfizer-BioNTech or Moderna (COVID-19 mRNA vaccines) are preferred. You may get Johnson & Johnson's Janssen COVID-19 vaccine in some situations.
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
Are antibiotics effective in preventing or treating COVID-19?
Antibiotics do not work against viruses; they only work on bacterial infections. Antibiotics do not prevent or treat COVID-19, because COVID-19 is caused by a virus, not bacteria. Some patients with COVID-19 may also develop a bacterial infection, such as pneumonia.Mar 31, 2022
How can convalescent plasma be used to treat COVID-19?
The blood from people who recover from COVID-19 contains substances called antibodies, which are capable of fighting the virus that causes the illness. For some other diseases caused by respiratory viruses, giving people the liquid portion of blood that contains these antibodies, called plasma, obtained from those who have recovered from the virus, may lead to more rapid improvement of the disease. Patients with COVID-19 may improve faster if they receive plasma from those who have recovered from COVID-19, because it may have the ability to fight the virus that causes COVID-19.Dec 28, 2021
Should you still get the COVID-19 vaccine if you were treated with monoclonal antibodies?
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, there is no need to delay getting a COVID-19 vaccine.Feb 17, 2022
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
How do monoclonal antibodies work against cancer?
Monoclonal antibodies are immune system proteins that are created in the lab. Antibodies are produced naturally by your body and help the immune sy...
Which cancers are treated with monoclonal antibodies?
Many monoclonal antibodies have been approved to treat a wide variety of cancers. To learn about specific treatments for your cancer, see the PDQ®...
What are the side effects of monoclonal antibodies?
Monoclonal antibodies can cause side effects, which can differ from person to person. The ones you may have and how they make you feel will depend...
WHAT IS A MONOCLONAL ANTIBODY?
Your body naturally makes antibodies to fight infection. However, your body may not have antibodies designed to recognize a novel (or new) virus like SARS-CoV-2, the virus that causes COVID-19.
How Can I Get Monoclonal Antibodies?
To receive a mAb you should be referred for treatment by your healthcare professional and directed to available infusion locations. If you do not have a healthcare provider, call the Combat COVID Monoclonal Antibodies Call Center at 1-877-332-6585 to find out who to talk with about your symptoms and treatment.
WHAT IF I DO NOT QUALIFY FOR MONOCLONAL ANTIBODY TREATMENT?
Your healthcare professional may decide you do not qualify for mAb treatment. There could be several reasons for this. You may not meet all eligibility criteria or you may have an underlying health condition that disqualifies you for mAb treatment.
WHAT CAN I EXPECT FROM TREATMENT (INFUSION)?
The mAb treatment is usually offered at an infusion center because the treatment is given through an intravenous (IV) infusion or shots. Depending on the mAb treatment you receive, the whole process takes about 1-3 hours, depending on the treatment..
CAN MONOCLONAL ANTIBODY TREATMENT MAKE ME SICK?
Antibody treatments do not contain any live SARS-CoV-2, so there is no risk you will get COVID-19 from mAb treatment. However, the antibody treatment may have side effects:
What antibody is used to block the virus?
Monoclonal antibodies against COVID-19 attach to the virus to block it from entering human cells. The monoclonal antibody protein also “marks” the virus to be broken down by the immune system and cleared from the body.
What is the function of antibodies?
Antibodies are proteins that exist in our bodies as part of our immune system to recognize and defend against harmful viruses and bacteria. Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
Can monoclonal antibodies cause nausea?
Most people tolerate monoclonal antibody infusions very well. Some people may experience infusion-related side effects, such as nausea and dizziness, that are short-lived and go away on their own. As with any medication, there is the potential for mild or more severe allergic reactions, which are uncommon.
COVID-19 VEKLURYTM (remdesivir)
Following the recent statement from the National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel about therapies for the COVID-19 Omicron variant, CMS created HCPCS code J0248 for VEKLURY™ (remdesivir) antiviral medication when administered in an outpatient setting.
COVID-19 Monoclonal Antibody Products
The FDA authorized the following investigational monoclonal antibody product under EUA for pre-exposure prophylaxis of COVID-19:
Important Update about Viral Variants
On April 16, 2021, the FDA revoked the EUA for bamlanivimab, when administered alone , due to a sustained increase in COVID-19 viral variants in the U.S. that are resistant to the solo product.
Medicare Coverage for COVID-19 Monoclonal Antibody Products
During the COVID-19 public health emergency (PHE), Medicare will cover and pay for these infusions (when furnished consistent with their respective EUAs) the same way it covers and pays for COVID-19 vaccines.
Coding for the Administration of COVID-19 Monoclonal Antibody Products
CMS identified specific code (s) for each COVID-19 monoclonal antibody product and specific administration code (s) for Medicare payment:
Medicare Payment for Administering COVID-19 Monoclonal Antibody Products
To ensure immediate access during the COVID-19 PHE, Medicare covers and pays for these infusions and injections in accordance with Section 3713 of the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) .
Billing for Administering COVID-19 Monoclonal Antibody Products
Health care providers can bill on a single claim for administering COVID-19 monoclonal antibody products, or submit claims on a roster bill.
What is monoclonal antibody?
Monoclonal antibodies are immune system proteins that are created in the lab. Antibodies are produced naturally by your body and help the immune system recognize germs that cause disease, such as bacteria and viruses, and mark them for destruction.
Why are monoclonal antibodies used in immunotherapy?
Some monoclonal antibodies are also immunotherapy because they help turn the immune system against cancer. For example, some monoclonal antibodies mark cancer cells so that the immune system will better recognize and destroy them.
What antibodies kill cancer cells?
Other monoclonal antibodies bring T cells close to cancer cells, helping the immune cells kill the cancer cells. An example is blinatumomab (Blincyto®), which binds to both CD19, a protein found on the surface of leukemia cells, and CD3, a protein on the surface of T cells. This process helps the T cells get close enough to ...
Can cytokine release cause shock?
Capillary leak syndrome may lead to multiple organ failure and shock. Cytokine release syndrome can sometimes occur with monoclonal antibodies, but it is often mild. Cytokines are immune substances that have many different functions in the body, and a sudden increase in their levels can cause: Fever. Nausea.
Can monoclonal antibodies cause side effects?
Monoclonal antibodies can cause side effects, which can differ from person to person. The ones you may have and how they make you feel will depend on many factors, such as how healthy you are before treatment, your type of cancer, how advanced it is, the type of monoclonal antibody you are receiving, and the dose.
Overview
Monoclonal antibodies (also called moAbs or mAbs) are proteins made in laboratories that act like proteins called antibodies in our bodies. Antibodies are parts of your immune system. They seek out the antigens (foreign materials) and stick to them in order to destroy them.
Procedure Details
In most cases, monoclonal antibodies are given mostly as intravenous (IV) solution injected right into your vein (sometimes referred to as an infusion). They’re often given in an infusion center where there are several people getting treatment at one time.
Recovery and Outlook
Infusion times can vary. As an example, though, monoclonal antibody treatment for COVID-19 under Emergency Use Authorization took about an hour for infusion and then another hour or so to watch for any reaction to the infusion.
When to Call the Doctor
If you’ve had a monoclonal antibody treatment, and you’re having an expected reaction, call your healthcare provider or go to an emergency room.