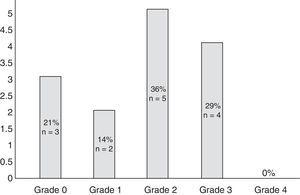
Bortezomib in the treatment of cancer Abstract Bortezomib (Velcade, formerly PS-341) represents the first proteasome inhibitor to have shown anti-tumor activity in both solid and haematological malignancies.
What is bortezomib (Velcade)?
Bortezomib is also called by its brand name Velcade. It is a treatment for myeloma and mantle cell lymphoma. You can have bortezomib on its own. Or with other cancer drugs such as:
What kind of cancer is bortezomib used for?
Bortezomib is approved to treat adults with: Mantle cell lymphoma in patients who have received at least one other type of treatment. Multiple myeloma. Bortezomib is also being studied in the treatment of other types of cancer.
What type of chemotherapy is Velcade?
VELCADE (bortezomib) is a type of chemotherapy called a targeted therapy. VELCADE belongs to a class of medicines called proteasome inhibitors. It is approved by the FDA for the treatment of multiple myeloma and mantle cell lymphoma.
Can Velcade be used to treat lymphoma off-label?
While Velcade is approved to treat certain types of lymphoma, it’s not approved to treat all forms of lymphoma. So in some cases, Velcade is used off-label to treat certain forms of lymphoma. Below, we describe certain lymphomas that Velcade is sometimes used off-label to treat.

What are the side effects of bortezomib?
SIDE EFFECTS: Dizziness, lightheadedness, nausea, vomiting, loss of appetite, diarrhea, constipation, tiredness, weakness, blurred vision, or pain/redness at injection site may occur. If any of these effects persist or worsen, notify your doctor or pharmacist promptly.
How long can you take bortezomib?
You can have bortezomib on its own if you have already had treatment for myeloma. You usually have bortezomib twice a week, for 2 weeks. Then you have a break of 10 days. This 3 week period is called a cycle of treatment.
Do you lose your hair with bortezomib?
Hair loss isn't a direct side effect of Velcade, but it may be a side effect of other drugs used with Velcade. For example, hair loss may occur in people using Velcade with cyclophosphamide, which is a cytotoxic drug. (Cytotoxic drugs work by killing cells.)
What are the side effects of the medication Velcade?
Side effects of VELCADE include:Fatigue. ... Peripheral neuropathy. ... Low blood pressure (hypotension) ... Heart problems. ... Lung disorders or problems. ... Liver disease or problems. ... Hematologic disease (Thrombotic Microangiopathy, TMA) ... Posterior reversible encephalopathy syndrome (PRES)More items...
Is VELCADE considered chemotherapy?
VELCADE (bortezomib) is a type of chemotherapy called a targeted therapy. VELCADE belongs to a class of medicines called proteasome inhibitors. It is approved by the FDA for the treatment of multiple myeloma and mantle cell lymphoma. VELCADE has been studied in many important clinical trials.
How expensive is VELCADE?
The cost for Velcade injectable powder for injection 3.5 mg is around $1,697 for a supply of 1 powder for injection, depending on the pharmacy you visit. Prices are for cash paying customers only and are not valid with insurance plans.
How does Velcade make you feel?
Dizziness, lightheadedness, nausea, vomiting, loss of appetite, diarrhea, constipation, tiredness, weakness, or pain/redness at the injection site may occur. Nausea, vomiting, and diarrhea can be severe. In some cases, your doctor may prescribe medication to prevent or relieve nausea, vomiting, and diarrhea.
How effective is Velcade?
In a three-drug study, 85 percent of patients responded well to the treatment plan and 40 percent of patients experienced remission after one to two years. Similar success was achieved with a four-drug combination—96 percent of patients responded to the treatment and 39 percent went into remission.
How long is Velcade treatment?
VELCADE is given intravenously twice a week for 2 weeks followed by a 10-day rest period. This can be repeated for up to 6 cycles.
Does VELCADE cause hearing loss?
Long-term risks related to the toxicity of the conditioning therapy and the consequently altered immunological status may include bilateral hearing impairment (high tone deficiency). These risks deserve long-term care following intensive regimens.
How often is VELCADE given?
VELCADE is administered twice weekly for two weeks (Days 1, 4, 8, and 11) followed by a ten day rest period on Days 12 to 21.
How long is VELCADE treatment for multiple myeloma?
Velcade is given for nine 6-week treatment cycles.
How does bortezomib work?
How it works. Bortezomib is a type of cancer treatment drug called a proteasome inhibitor. Proteasomes are in cells. They help to break down proteins that the cell doesn't need. Bortezomib blocks the proteasomes so the proteins build up inside the cell. The cell then dies.
How long can you take Bortezomib?
When you have it. You can have bortezomib on its own if you have already had treatment for myeloma. You usually have bortezomib twice a week, for 2 weeks. Then you have a break of 10 days. This 3 week period is called a cycle of treatment. The length of your treatment cycle might be different.
What is the name of the drug that is used to treat myeloma?
Bortezomib is also called by its brand name Velcade. It is a treatment for myeloma and mantle cell lymphoma.
What do they check before and during a chemo treatment?
You have blood tests before and during your treatment. They check your levels of blood cells and other substances in the blood. They also check how well your liver and kidneys are working.
Does Bortezomib cause serum M levels to go down?
Serum M is made by myeloma cells. So if the bortezomib is working, the serum M levels go down. You have a further 2 cycles of treament if all signs of the myeloma disappear. Or you have 8 cycles if the myeloma does not completely disappear.
What is velcade injection?
VELCADE is an injection that is given intravenously (into your vein) or subcutaneously (under your skin) by a healthcare professional.
What is velcade used for?
VELCADE belongs to a class of medicines called proteasome inhibitors. It is approved by the FDA for the treatment of multiple myeloma and mantle cell lymphoma. VELCADE has been studied in many important clinical trials. It has been studied in a wide range of people, including those with renal impairment and diabetes.
What is the treatment for mantle cell lymphoma?
In a clinical trial, people with mantle cell lymphoma received either VELCADE or vincristine along with rituximab, cyclophosphamide, doxorubicin, and prednisone
How does Velcade work?
As a targeted therapy, VELCADE works by blocking or slowing down the action of proteasomes inside cells. The function of proteasomes is to break down proteins in both healthy and cancerous cells.
How long does Velcade therapy last?
In a study, half of the people taking VELCADE-based therapy stayed on VELCADE for at least 50 weeks out of a planned 54 weeks
How long does it take to take Velcade?
VELCADE is given once a week for 2 weeks, followed by a 13-day rest period. This sequence is then repeated to make one 6-week cycle. There are 5 cycles in the second treatment phase. You must wait at least 3 days between VELCADE doses.
How long does it take for Velcade to respond to treatment?
In this study of VELCADE, in previously untreated people, some people had their best response as early as 6 weeks. Others continued to respond after 6 months of treatment. If you don’t respond early, don’t be discouraged. It’s important to stick with your treatment plan if you and your doctor decide it’s right for you.
How is VELCADE administered?
It is administered by a healthcare professional as an injection into your vein (intravenously, or IV) or under your skin (subcutaneously, or SC).
What are the possible side effects of VELCADE?
Tell your healthcare provider if you get any new or worsening symptoms, including: muscle weakness, tingling, burning, pain, and loss of feeling in your hands and feet, any of which can be severe. Your doctor may change the dose and/or schedule of VELCADE or stop it altogether. If you have peripheral neuropathy before starting VELCADE, your doctor could consider giving you VELCADE subcutaneously.
What should I avoid while taking VELCADE?
VELCADE may cause fatigue, dizziness, fainting (syncope), or lightheadedness when you sit or stand up. You should not drive or operate machinery if you experience any of these symptoms.
How long after velcae can you get pregnant?
Females should use effective birth control during treatment and for at least seven months after the final dose of VELCADE. If using hormonal contraceptives (for example, the pill), an additional barrier method of contraception (for example, diaphragm or condom) must be used. Males should use effective contraception during treatment with VELCADE and for four months following treatment. Tell your doctor immediately if you think you are pregnant. Do not breastfeed during treatment with VELCADE and for two months after your final dose of VELCADE.
Can velcade be administered to spinal fluid?
It is administered by a healthcare professional as an injection into your vein (intravenously, or IV) or under your skin (subcutaneously, or SC). VELCADE must not be administered into your spinal fluid (intrathecally).
Can you restart Velcade?
It is not known whether restarting VELCADE therapy in patients previously experiencing this complication is safe. Hematologic disease (Thrombotic Microangiopathy, TMA). VELCADE can lead to the formation of blood clots in small blood vessels.
Can Velcade cause lung problems?
Lung problems (pulmonary toxicity). There have been reports of lung disorders in people receiving VELCADE. Some of these events have been fatal. Tell your doctor if you experience any cough, shortness of breath, wheezing, or difficulty breathing.
How is bortezomib given?
Bortezomib can be given into a vein (IV, intravenous) or an injection under the skin (subcutaneous). The actual dose is based on your body size and the schedule is dependent on the type of cancer you have.
How to deal with fatigue after cancer treatment?
While on cancer treatment, and for a period after, you may need to adjust your schedule to manage fatigue. Plan times to rest during the day and conserve energy for more important activities. Exercise can help combat fatigue; a simple daily walk with a friend can help. Talk to your healthcare team for helpful tips on dealing with this side effect.
How to help nausea and vomiting from oncology?
Talk to your oncology care team so they can prescribe medications to help you manage nausea and vomiting. In addition, dietary changes may help. Avoid things that may worsen the symptoms, such as heavy or greasy/fatty, spicy or acidic foods (lemons, tomatoes, oranges). Try saltines, or ginger ale to lessen symptoms.
Why is WBC important?
White blood cells (WBC) are important for fighting infection. While receiving treatment, your WBC count can drop, putting you at a higher risk of getting an infection. You should let your doctor or nurse know right away if you have a fever (temperature greater than 100.4°F or 38°C), sore throat or cold, shortness of breath, cough, burning with urination, or a sore that doesn't heal.
Does green tea affect bortezomib?
Certain medications and foods, including green tea, ketoconazole, rifampin, and St. John’s Wort, can interfere with blood levels of bortezomib. Make sure your provider is aware of all the medications, vitamins, and supplements you are taking.
What is Velcade?
Velcade (bortezomib) interferes with the growth of some cancer cells and keeps them from spreading in your body.
How long does Velcade last?
In cycles 5 through 9, Velcade is administered once weekly (days 1, 8, 22, and 29).#N#Comments:#N#-At least 72 hours should elapse between consecutive doses of Velcade.#N#FOR USE IN THE TREATMENT OF RELAPSED MULTIPLE MYELOMA:#N#-Usual dose: 1.3 mg/m2 as a bolus intravenous injection or subcutaneously twice weekly for two weeks (days 1, 4, 8, and 11) followed by a ten day rest period (days 12 through 21). Therapy extending beyond 8 cycles may be administered by the standard schedule or may be given once weekly for 4 weeks (days 1, 8, 15, and 22), followed by a 13-day rest (days 23 through 35).#N#Comments:#N#-Velcade may be administered alone or in combination with dexamethasone.#N#-The three week period is considered a treatment cycle.#N#-A minimum of 72 hours should elapse between consecutive doses of Velcade.#N#-Patients with multiple myeloma who have previously responded to treatment with Velcade (either alone or in combination) and who have relapsed at least 6 months after their prior therapy may be started on the last tolerated dose.#N#Use: For the treatment of multiple myeloma (who had previously responded to treatment with this drug and who have relapsed at least 6 months after completing treatment)
What should I avoid while receiving Velcade?
Avoid becoming dehydrated if you have any vomiting or diarrhea. Talk with your doctor about how best to keep yourself hydrated.
What other drugs will affect Velcade?
Sometimes it is not safe to use certain medications at the same time. Some drugs can affect your blood levels of other drugs you take, which may increase side effects or make the medications less effective.
What are the risks of Velcade?
To make sure Velcade is safe for you, tell your doctor if you have ever had: 1 nerve problems such as numbness, tingling, or burning pain; 2 diabetes; 3 liver disease; 4 kidney disease, or if you are on dialysis; 5 a low level of platelets or white or red blood cells; 6 heart disease, congestive heart failure; 7 lung disease or breathing problems; 8 herpes or shingles ( herpes zoster ); 9 high or low blood pressure; or 10 if you are dehydrated.
What to do if you miss your appointment for Velcade?
Call your doctor for instructions if you will miss an appointment for your Velcade injection.
How often can you take Velcade?
Therapy extending beyond 8 cycles may be administered by the standard schedule or may be given once weekly for 4 weeks (days 1, 8, 15, and 22), followed by a 13-day rest (days 23 through 35). Comments: -Velcade may be administered alone or in combination with dexamethasone.
How is Velcade given?
Velcade is given by either intravenous (IV) injection (an injection into your vein) or subcutaneous injection (an injection under your skin). You’ll receive doses of Velcade from a healthcare provider at a medical facility.
What is Velcade a class of drugs?
Velcade contains the active drug bortezomib. It belongs to a class of drugs called proteasome inhibitors. (A class of drugs is a group of medications that work in the same way.) Velcade works by inhibiting (blocking) the action of certain proteins called proteasomes.
How long do side effects from Velcade last?
Velcade’s side effects will likely affect each person a little differently. And how long certain side effects of Velcade last will vary from person to person . If you have side effects from Velcade, talk with your doctor about how long you can expect them to last. Your doctor may be able to advise whether certain side effects will go away over time.
How long does it take to get Velcade for multiple myeloma?
If you have untreated multiple myeloma, you’ll likely receive Velcade over nine 6-week treatment cycles. And Velcade is typically given with the chemotherapy drug melphalan and the steroid prednisone.
How long does it take for Velcade to work?
Velcade may start working within 5 minutes after it’s been given to you. However, you may not notice that Velcade is working. This is because the drug works to stop your cancer from worsening. But it may not reduce symptoms you’re having from your cancer.
How much Velcade is in a vial?
Velcade comes as a powder that’s mixed with liquid to form a solution. It’s available in one strength: 3.5 milligram (mg) of drug per vial of medication.
Does Velcade cause a rash?
11% of people taking Velcade with melphalan and prednisone had a rash. (Melphalan and prednisone are other drugs that are sometimes used in multiple myeloma treatment.) 2% of people taking melphalan and prednisone alone had a rash. Another study looked at people with relapsed multiple myeloma.
What is Velcade anticancer?
Velcade® is the first drug of its kind in a new class of anticancer drugs called proteasome inhibitors. A collaboration between the National Cancer Institute (NCI) and ProScript, a biotech company, led to the initial clinical trial of Velcade® and to approval by the Food and Drug Administration (FDA) for treatment of MM.
What is Velcade used for?
Velcade® ( bortezomib ), originally known as PS-341, was first developed by ProScript, a biotech company, to treat muscle weakness and muscle loss associated with AIDS and muscular dystrophy (a group of more than 30 inherited diseases that cause muscle weakness and muscle loss). Julian Adams, Ph.D., a ProScript chemist, ...
What is the new class of anticancer drugs?
This new approach to destroying cancer helped to develop an entirely new class of anticancer drugs called proteasome inhibitors. He and his fellow scientists at ProScript continued to refine PS-341 as a proteasome inhibitor that was highly specific and effective enough to block the activity of the proteasome.
Who discovered Velcade?
The discovery of Velcade® for the treatment of MM originated from a collaboration with ProScript Inc. and NCI scientists, Drs. Edward A. Sausville and Jill Johnson from the Division of Cancer Treatment and Diagnosis, Developmental Therapeutics Program. NCI has supported and continues to fund research on the structural and biochemical complexities of the proteasome, as well as clinical trials on Velcade ® and various drug combinations.
When was PS-341 approved?
In 2003, PS-341 was approved by the FDA to treat MM and was branded with the official name bortezomib (Velcade®). The drug was initially approved only for patients whose cancer did not respond to conventional treatments or came back after initially responding to treatment.
When did PS-341 go into remission?
In 2000, in a phase I clinical trial, while giving a very low dose of PS-341 to a patient with multiple myeloma to test for safety, researchers were stunned to find that the patient's cancer went into complete remission.
Does Velcade cause pain?
These abnormal plasma cells stimulate the breakdown of actively used bones such as the spine, pelvis, and bones around the shoulders and hips. This can cause extreme pain. Prior to the development of Velcade®, no effective treatment options were available for MM.
