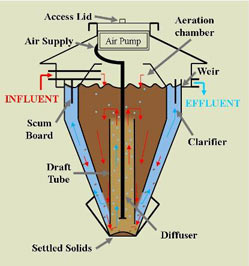
Significance of BOD
- BOD finds its primary importance in sewage treatment plants. ...
- It determines the rate of respiration in living beings.
- Measuring BOD gives the COD or Chemical Oxygen Demand of inorganic substances.
- It indicates the polluting potential of water.
- BOD is used in medical and pharmaceutical industries to measure the oxygen consumption of cell cultures.
What is bod in wastewater treatment?
Oct 30, 2018 · What Is Biological Oxygen Demand in Wastewater Treatment? Biochemical oxygen demand or biological oxygen demand (BOD) is a measure of the amount of Dissolved Oxygen (DO) required by aerobic microorganisms to decompose organic matter present in a sample of water at a certain temperature over a studied period.
What is BOD5 test in wastewater treatment?
Industries that discharge wastewater into municipal sanitary sewers or waterways are facing strict regulations on levels of biological or biochemical oxygen demand (BOD). Solid materials in wastewater can consist of organic and/or inorganic materials and organisms. The solids must be significantly reduced by treatment or they can increase BOD when discharged. ALAR Water …
What is BOD of water?
Sep 06, 2021 · Among the most important components of the wastewater treatment process involves biological oxygen demand. BOD refers to the total amount of oxygen that bacteria and other forms of microorganisms will consume while decomposing any organic matter that’s present in the water.
What is BOD and cod in water pollution?
Nov 13, 2018 · The waste organic matter is stabilized or made unobjectionable through its decomposition by living bacterial organisms which need oxygen to do their work. BOD is used, often in wastewater-treatment plants, as an index of the degree of organic pollution in water.
What is the BOD of treated water?
The biochemical oxygen demand for treated water should be zero. Since it represents the amount of biodegradable organic matter present in water which is food for bacteria or microorganism, if not treated can cause several water-borne diseases.
What is BOD and Its Significance in sewage treatment?
Importance of BOD – Biochemical oxygen demand It indicates the amount of organic pollution present in an aquatic ecosystem. BOD also measures the chemical oxidation (COD) of inorganic matter. BOD is also used in sewage treatment or wastewater treatment to destroy and decay organic wastes through the aerobic organisms.
What causes high BOD in wastewater?
BOD represents the amount of organic matter in a water supply; therefore, it increases when decaying plants, human or animal waste, and other organic compounds are added to water.Mar 12, 2020
What will be the BOD value in sewage?
The amount of oxygen required by bacteria to break down the organic matter present in a certain volume of a sample of water, is called Biochemical Oxygen Demand (BOD). It is measured in ppm. The BOD of municipal sewage is 100-4000 ppm.
Why BOD test is done?
Biochemical oxygen demand, or BOD, is a chemical procedure for determining the amount of dissolved oxygen needed by aerobic biological organisms in a body of water to break down organic material present in a given water sample at certain temperature over a specific time period.
What is BOD and COD Byjus?
Biochemical Oxygen Demand (BOD) is the amount of oxygen that is dissolved and consumed by biological organisms when they decompose organic matters in water bodies. Chemical Oxygen Demand (COD) is the amount of oxygen that is consumed when a water sample is chemically oxidised.
Does chlorine reduce BOD?
BOD reduction: Chlorine accomplishes BOD reduction by oxidation of organic compounds present in wastewaters. 4. Oxidation of metal ions: Metal ions which are in reduced state are oxidized by chlorine (e.g., ferrous to ferric ion and manganous to manganic ions).
What happens if BOD is low?
The dissolved oxygen readings are usually in parts per million (ppm). Higher BOD indicates more oxygen is required, which is less for oxygen-demanding species to feed on, and signifies lower water quality. Inversely, low BOD means less oxygen is being removed from water, so water is generally purer.
What happens if BOD is high?
The greater the BOD, the more rapidly oxygen is depleted in the stream. This means less oxygen is available to higher forms of aquatic life. The consequences of high BOD are the same as those for low dissolved oxygen: aquatic organisms become stressed, suffocate, and die.
What is a good BOD level?
Typical values Most pristine rivers will have a 5-day carbonaceous BOD below 1 mg/L. Moderately polluted rivers may have a BOD value in the range of 2 to 8 mg/L. Rivers may be considered severely polluted when BOD values exceed 8 mg/L.
What is difference between BOD and COD?
Biochemical oxygen demand (BOD) is the amount of oxygen required by the microorganisms to break down the organic materials, whereas chemical oxygen demand (COD) is the amount of oxygen required to break down the organic material via oxidation.
What is BOD COD ratio?
BOD is oxygen demand for oxidation of organic matter by micro organism. COD is oxygen demand for oxidation of organic and non biodegradable organic matter. COD is always greater than BOD hence the ratio will be always greater than 1. If BOD/COD is 0.92 to 1, waste water is fully biodegradable.
What is BOD in wastewater?
Industries that discharge wastewater into municipal sanitary sewers or waterways are facing strict regulations on levels of biological or biochemical oxygen demand (BOD). Solid materials in wastewater can consist of organic and/or inorganic materials and organisms.
What is the purpose of biological oxygen demand?
Biological Oxygen Demand (BOD) is a chemical procedure for determining the amount of dissolved oxygen needed by aerobic biological organisms “bio-bugs” in a body of water to break down organic material present in a given water sample at a certain temperature over a specific time period.
What is biological oxygen demand?
Biochemical Oxygen Demand or Biological Oxygen Demand, is a measurement of the amount of dissolved oxygen (DO) that is used by aerobic microorganisms when decomposing organic matter in water. BOD can be measured in real-time with our BOD sensors to improve wastewater process control and plant efficiency.
Why is biological oxygen demand important?
Biochemical oxygen demand / biological oxygen demand is an important water quality parameter because it provides an index to assess the effect discharged wastewater will have on the receiving environment. The higher the BOD value, the greater the amount of organic matter or “food” available for oxygen consuming bacteria. If the rate of DO consumption by bacteria exceeds the supply of DO from aquatic plants, algae photosynthesis or diffusing from air, unfavourable conditions occur. Depletion of DO causes stress on aquatic organisms, making the environment unsuitable for life. Further, dramatic depletion can lead to hypoxia or anoxic environments. BOD is also used extensively for wastewater treatment, as decomposition of organic waste by microorganisms is commonly used for treatment.
What is BOD5 in wastewater?
Typically, municipal wastewater treatment plants will use BOD5 as a measure of the organic concentration into, and through, the wastewater plant. Industrial wastewater systems will more often use COD to measure the organic concentration moving through the treatment plant. In my experience, I see TOC being used much less often (rarely) ...
What is the BOD5 test?
The BOD5 test measures the oxygen consumed by microorganisms as they oxidize (consume or eat) the soluble organic matter in the wastewater. But the BOD5 test is a somewhat unreliable means of determining the amount of organic matter present in water. The test measures only the approximate amount of oxygen that will be required ...
How long does it take to test for organics in wastewater?
The often highly variable chemical composition and strength of industrial wastewater requires a much more rapid method for measuring the organic concentration, hence the use of the two hour COD test or, in some plants, the 30 minute TOC analysis.
What is the purpose of a wastewater test?
The test measures only the approximate amount of oxygen that will be required (absorbed or consumed) by a wastewater when it is exposed to air or oxygen for an extended period of time. Toxic substances in the wastewater inhibit or even prevent bacterial growth and, therefore, oxidation of the organic matter.
How to reduce BOD in water?
The lesser we contaminate the water bodies lesser is its biochemical oxygen demand levels. There are various treatments done to reduce BOD levels in various wastewaters. The secondary effluent treatment is done in the sewage treatment plants to reduce the BOD in the sewage wastewater. The continuous air supply is maintained. There are other treatments like flocculation, coagulation, the addition of chemicals like hydrogen peroxide that oxidized organic waste. Addition of ozone, various reagents, the passing of UV rays etc.
What is the purpose of BOD?
BOD is used as an index for measuring water quality. Determining organic matter present in a water body and its effect on the ecosystem and aesthetics of the water body is an integral part of water quality management.
Why is it important to reduce the biochemical oxygen demand of water bodies before discharging into water bodies?
It is extremely important to reduce the biochemical oxygen demand of water bodies or wastewater before discharging into water bodies because high BOD of water means more and more oxygen is utilized by the aerobic bacteria for breaking down the organic waste in the water. This significantly reduces the available oxygen for the respiration ...
What is the biological oxygen demand?
Biochemical Oxygen Demand or alternatively termed as Biological Oxygen Demand (BOD) is the amount of oxygen needed or demanded by aerobic microorganisms to break down the organic matter present in a certain sample of water at a specific temperature and over a given time period. Water bodies have a certain amount of oxygen dissolved in it on which ...
How is oxygen demand measured in water?
Biochemical Oxygen Demand of a water sample is measured by a Bioassay procedure which measures the oxygen consumed by the bacteria from the decomposition of the organic matter over a period of five days at an incubation temperature of 20°C. BOD is expressed in milligrams per litre of sample water. Although this is not a precise quantitative test, it is widely used as an indication of the polluting potential of water. This test was given by Sawyer and McCarty in the year 1978.
What is the BOD of water?
Ans. BOD or Biochemical Oxygen Demand is the measure of the amount of oxygen utilized by aerobic microbes to degrade the organic waste present in water. It is calculated over five days at a specific temperature of 20°C.
How does pollution affect the BOD of water?
Sources that increase Biological Oxygen Demand of water are both natural and man-made. Pollution is a major contributor to increasing the BOD of water bodies. A good lifestyle is associated with an ample usage of water on a regular basis which results in a lot of wastewater with organic content in it. With increasing industrialization, pollution is increasing manifold. Factories have enormous wastewater being generated. Few industries that have huge quantities of wastewater are paper mills, food processing plants, jute mills, etc. The environmental factors contributing to increasing BOD include surface runoff, floating debris, dead animal and plants, soil erosion, etc. There are few chemicals that affect the BOD of drinking water. One of these is phosphate, which when present in high amount increases the BOD of water.
What is COD in water?
COD, the chemical demand for oxygen a source of water has, is the amount of oxygen required to break down organic substances chemically and convert them to CO 2 and H 2 O. It is also expressed in mgO 2 /L, and the higher the COD, the more polluted the water is.
How much COD is in water?
The COD in industrial water may be 50 – 2,000 mgO 2 /L, although it may reach 5,000, depending on the type of industry. The main difference between BOD and COD is that COD measures all organic material, while BOD only measures organic material which is or can be biologically degraded.
What is the purpose of COD?
In environmental chemistry, the chemical oxygen demand (COD) test is commonly used to indirectly measure the amount of organic compounds in water. Most applications of COD determine the amount of organic pollutants found in surface water (e.g. lakes and rivers), making COD a useful measure of water quality.
What is COD in biology?
Chemical Oxygen Demand (COD): COD is defined as the oxygen equivalent of the organic portion of the sample that is susceptible to oxidation by a strong chemical oxidant potassium dichromate K2Cr2O7, Biochemical Oxygen Demand (BOD) is a chemical procedure for determining how fast biological organisms use up oxygen in a body of water.
What is the purpose of the Biochemical Oxygen Demand?
Biochemical Oxygen Demand (BOD) is a chemical procedure for determining how fast biological organisms use up oxygen in a body of water. It is usually performed over a5-day period at20° Celsius. It is used in water quality management and assessment, ecology and environmental science.
Is BOD a pollutant?
It is listed as a conventional pollutant . BOD is similar in function to chemical oxygen demand (COD), in that both measure the amount of organic compounds in water. However, COD is less specific, since it measures everything that can be chemically oxidized, rather than just levels of biologically active organic matte.