
Full Answer
What is the best antigen?
Immunology 101 states that the immune system does many things but the single-minded focus of the cell-mediated, TH1 response (more on this later) is to KILL the foreign invader – AKA the ANTIGEN. In autoimmune disease, the immune system is killing self-cells but it …
What is the initial exposure to an antigen?
Apr 01, 2015 · What is the Antigen and How are we going to get RID of the antigen? You will learn that, by definition, an autoimmune disease is a disorder where your immune system is creating antibodies of self-tissue. Whatever you have antibodies for, be it a bacteria or your thyroid tissue, your killer side of your immune system will destroy it.
What is the relationship between an antigen and an antibody?
Apr 01, 2022 · An antigen is any substance that causes your immune system to produce antibodies against it. This means your immune system does not recognize the substance, and is trying to fight it off. An antigen may be a substance from the environment, such as chemicals, bacteria, viruses, or pollen. An antigen may also form inside the body.
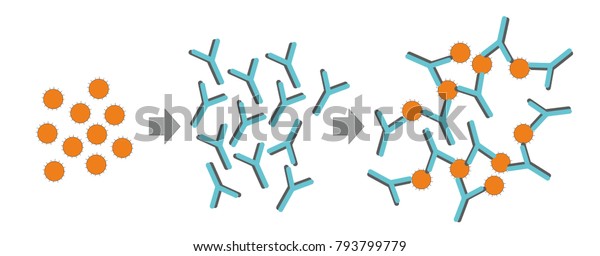
How can an antibody recognize an antigen?
Mar 24, 2022 · Antigen Testing for SARS-CoV-2 Infection Antigen-based diagnostic tests (which detect viral antigens) are less sensitive than RT-PCR-based tests, but they have similarly high specificity. Antigen tests perform best early in the course of symptomatic SARS-CoV-2 infection, when the viral load is thought to be highest.
What is a monoclonal antibody for COVID-19?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells. Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Who could benefit from monoclonal antibody therapy to prevent COVID-19?
See full answerVaccines are the best way to protect against COVID-19. But some people with weakened immune systems do not produce enough antibodies after vaccination, and others are severely allergic to the vaccine. The FDA recently authorized Evusheld, a pre-exposure prophylaxis (PrEP) monoclonal antibody therapy developed by AstraZeneca, which should help prevent COVID-19 in these populations.To be eligible for Evusheld, individuals must be 12 years or older and have a moderately to severely weakened immune system, or have a history of severe adverse reactions to the COVID-19 vaccine or its components. In addition, the therapy cannot be given to someone with a current SARS-CoV-2 infection, or who has been recently exposed to someone who is infected. Evusheld is given as two consecutive shots, and evidence suggests it can help prevent symptomatic infection for at least six months.Apr 1, 2022
Can you get COVID-19 if you already had it and have antibodies?
It is important to remember that some people with antibodies to SARS-CoV-2 may become infected after vaccination (vaccine breakthrough infection) or after recovering from a past infection (reinfected).Nov 10, 2021
What is the first drug that was approved by the FDA to treat COVID-19?
Remdesivir is the first drug approved by the FDA for treatment of hospitalized COVID patients over the age of 12.Jan 25, 2022
Which drug is approved by FDA to treat COVID-19?
Veklury (Remdesivir) is an antiviral drug approved for use in adults and pediatric patients [12 years of age and older and weighing at least 40 kilograms (about 88 pounds)] for the treatment of COVID-19 requiring hospitalization.Mar 31, 2022
Are antibodies beneficial during the COVID-19 pandemic?
When reinfections or breakthrough infections happen, having antibodies plays an important role in helping prevent severe illness, hospitalization, and death. For many diseases, including COVID-19, antibodies are expected to decrease or “wane” over time.Nov 10, 2021
Who might benefit from dexamethasone if they have COVID-19?
Dexamethasone is a corticosteroid used in a wide range of conditions for its anti-inflammatory and immunosuppressant effects.It was tested in hospitalized patients with COVID-19 in the United Kingdom’s national clinical trial RECOVERY and was found to have benefits for critically ill patients.Oct 16, 2020
What do antibodies do to protect against COVID-19?
Antibodies are specialized proteins that are part of your immune system. They help protect against viruses, bacteria and other foreign substances. In the case of COVID-19, after you're infected with the SARS-CoV-2 virus, your immune system recognizes the virus as a foreign substance and forms antibodies against it.Nov 10, 2021
How long do COVID-19 antibodies last?
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.Jan 31, 2022
How long does it take to develop immunity after a COVID-19 infection?
Although the immune correlates of protection are not fully understood, evidence indicates that antibody development following infection likely confers some degree of immunity from subsequent infection for at least 6 months.
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
What are the advantages of antigen based diagnostic tests?
Advantages of antigen-based tests are their low cost and rapid turnaround time. The availability of immediate results makes them an attractive option for point-of-care testing in high-risk congregate settings where preventing transmission is critical. Antigen-based tests also allow for repeat testing to quickly identify persons with SARS-CoV-2 infection.
Why are there false positive results in serologic assays?
False positive test results may occur due to cross-reactivity from pre-existing antibodies to other coronaviruses.
What is the EUA test?
Two types of serologic tests have received EUAs from the FDA. The first type are antibody tests that detect the presence of binding antibodies, which bind to a pathogen (e.g., a virus). The second type of tests detect neutralizing antibodies from recent or prior SARS-CoV-2 infection.
What is a RT-PCR test?
Reverse transcriptase polymerase chain reaction (RT-PCR)-based diagnostic tests (which detect viral nucleic acids) are considered the gold standard for detecting current SARS-CoV-2 infection. More recently, NAATs have included a variety of additional platforms (e.g., reverse transcriptase loop-mediated isothermal amplification [RT-LAMP]). Clinically, there may be a window period of up to 5 days after exposure before viral nucleic acids can be detected. Diagnostically, some NAATs may produce false negative results if a mutation occurs in the part of the virus’ genome that is assessed by that test. 3 The FDA monitors the potential effects of SARS-CoV-2 genetic variations on NAAT results and issues updates when specific variations could affect the performance of NAATs that have received EUAs. Generally, false negative results are more likely to occur when using NAATs that rely on only one genetic target. Therefore, a single negative test result does not exclude the possibility of SARS-CoV-2 infection in people who have a high likelihood of infection based on their exposure history and/or their clinical presentation. 4
Is there a diagnostic test for SARS?
A number of diagnostic tests for SARS-CoV-2 infection (e.g., NAATs, antigen tests) have received Emergency Use Authorizations (EUAs) from the Food and Drug Administration (FDA), 1 but no diagnostic test has been approved by the FDA. Although nasopharyngeal specimens remain the recommended samples for SARS-CoV-2 diagnostic testing, ...
How does the COVID-19 antigen test work?
An antigen is a substance (protein) that causes the immune system to produce antibodies and trigger an immune response. In the case of COVID-19, spike proteins are found on the surface of SARS -CoV-2 virus. Therefore, the antigen test detects these proteins or genetic material of the SARS-CoV-2 virus.
How to use an at-home COVID-19 antigen test
The COVID-19 antigen test is affordable and may allow people to test themselves for COVID-19 rather than relying on getting tested at a screening center. The test includes all the necessary material for its use in nasopharyngeal samples:
What are the pros and cons of the COVID-19 antigen test?
One of the benefits of antigen testing is the high speed of testing compared with the standard reverse transcription polymerase chain reaction (RT-PCR).
What other tests are used to detect COVID-19?
Reverse transcription polymerase chain reaction (RT-PCR) is the gold standard of diagnostic testing for COVID-19.
Definition
An antigen is a piece of a virus that can be found in the blood and often causes the immune system to respond to an infection. The Australia antigen is a specific antigen used to diagnose patients with the hepatitis B virus. It got its name because it was first found in an Australian aborigine, but now we know it appears in anyone with hepatitis B.
Hepatitis B
Hepatitis B can be characterized as acute or chronic. Acute hepatitis B occurs shortly after infection and can often be cured during this period. Conversely, chronic hepatitis B is an infection that lasts longer than six months. At this point, it is unlikely the patient will ever be completely cured.
Symptoms
Not all people with the Australia antigen develop symptoms of hepatitis B. However, if symptoms of hepatitis B occur, they most often include a loss of appetite, fatigue, nausea and vomiting, localized pain in the mid-section near the liver, itching, jaundice, dehydration, and discolored urine and fecal matter.
Antibody Treatment
REGEN-COV, commonly referred to "antibody treatment" or "monoclonal antibody treatment," is a medicine used to treat mild to moderate symptoms of COVID-19 in non-hospitalized adults and children (12 years of age and older weighing at least 88 pounds) who are at high risk of developing severe COVID-19 symptoms or the need for hospitalization.
Important Information: Read before Scheduling!
You may be called by UTMB for additional eligibility screening before your scheduled appointment. We will call the number you provided when you schedule.
