
Amiodarone-induced thyrotoxicosis (AIT) is commonly due to iodine-induced excessive synthesis of thyroid, also known as type 1 AIT (AIT1). Amiodarone-induced thyrotoxicosis presenting as hypokalemic periodic paralysis. The effects of plasmapharesis on thyroid hormone and plasma drug concentrations in amiodarone-induced thyrotoxicosis.
Does amiodarone cause thyroid problems?
It is caused by antibodies that attack the thyroid and turn it on. Amiodarone: an iodine-rich drug that is commonly used for the treatment of irregular heart rhythms. Amiodarone can cause thyroid problems, including both hypothyroidism and hyperthyroidism.
How does amiodarone cause hypothyroidism?
It typically occurs in patients without underlying thyroid disease, and is caused by a direct toxic effect of amiodarone on thyroid follicular cells. The thyrotoxic phase may last several weeks to several months, and it is often followed by a hypothyroid phase with eventual recovery in most patients.
Does amiodarone cause tiredness?
unusual drowsiness, dullness, tiredness, weakness or feeling of sluggishness unusual excitement, nervousness, or restlessness vomiting of blood Side effects not requiring immediate medical attention Some side effects of amiodarone may occur that usually do not need medical attention.
How does amiodarone cause hyperthyroidism?
Amiodarone-induced thyrotoxicosis if a person with underlying thyroid disease is exposed to the high iodine content of amiodarone, this may lead to excess thyroid hormone synthesis and release. The long half-life of amiodarone may cause hyperthyroidism for up to one year after it is discontinued

How do you treat AIT?
Type 1 AIT should be treated with high doses of thioamides (20-60 mg/day of methimazole; or 400-600 mg/day of propylthiouracil) to block the synthesis of thyroid hormones (Figure 1). The response to thionamides is often modest due to the high iodine levels in patients taking amiodarone.
What is the treatment for thyrotoxicosis?
Medication – drugs called beta-blockers (e.g. propranolol), can be used to reduce the symptoms of thyrotoxicosis such as the heart rate, anxiety or sweating. However, to treat the raised hormone levels, different medication called carbimazole or another called propylthiouracil is used.
Why does amiodarone cause thyrotoxicosis?
Type I amiodarone-induced thyrotoxicosis In these patients, the sudden iodide load, associated with amiodarone treatment, accelerates thyroid hormone synthesis sufficiently to induce thyrotoxicosis because of increased thyroid hormone production in autonomous thyroid tissue (Jod—Basedow phenomenon) [Newman et al.
What is the target of amiodarone that can induce thyrotoxicosis as an adverse effect?
Amiodarone induces alterations in thyroid hormone levels by actions on thyroidal secretion, on the peripheral tissues, and probably also on the pituitary gland. These actions result in elevations in serum T4 and rT3 concentrations, transient increases in TSH concentrations, and decreases in T3 concentrations.
What is the difference between hyperthyroidism and thyrotoxicosis?
Hyperthyroidism is characterised by increased thyroid hormone synthesis and secretion from the thyroid gland, whereas thyrotoxicosis refers to the clinical syndrome of excess circulating thyroid hormones, irrespective of the source.
How long does it take to recover from thyrotoxicosis?
Thyroid function returns to normal within 12-18 months in 80% of patients. Painful subacute thyroiditis, the most common cause of thyroid pain, is a self limiting inflammatory disorder of possible viral aetiology.
Is amiodarone contraindicated in thyrotoxicosis?
Amiodarone inhibits entry of T4 and T3 into the peripheral tissue. Serum T4 levels increase by an average of 40% above pretreatment levels after 1-4 months of treatment with amiodarone. This, in itself, does not constitute evidence of hyperthyroidism (thyrotoxicosis).
Why does amiodarone cause thyroid level dysfunction?
However, amiodarone is associated with a number of side effects, including thyroid dysfunction (both hypo- and hyperthyroidism), which is due to amiodarone's high iodine content and its direct toxic effect on the thyroid.
What is thyrotoxicosis disease?
Thyrotoxicosis is a clinical state of inappropriately high levels of circulating thyroid hormones (T3 and/or T4) in the body from any cause[7]. It is often incorrectly used interchangeably with hyperthyroidism, which is a form of thyrotoxicosis caused by excessive endogenous thyroid hormone production.
Can amiodarone be used in thyroid storm?
Introduction: Thyroid storm is a rare medical emergency with high mortality, which usually results from acute exacerbation of hyperthyroidism. Amiodarone is a highly used class III antiarrhythmic drug and amiodarone-induced thyrotoxicosis is an infrequent effect of this medication, whose treatment may be difficult.
How long does amiodarone stay in your system?
Amiodarone has a very long half-life (this is the time it takes for 50% of a dose of amiodarone to be eliminated by the body) of 15 to 142 days. It also has an active metabolite, desethylamiodarone that has a half-life of 75 days.
How is amiodarone metabolized?
Amiodarone is metabolized in the liver via the cytochrome P450 system and hence increases the concentrations of medications such as statins, calcium channel blocking agents, quinidine, and flecainide. Amiodarone increases digoxin levels, hence concomitant digoxin should either be stopped or the dose reduced (50%) [Van Erven and Schalij, 2010]. Amiodarone inhibits the metabolism of coumadin (e.g. warfarin) derivates, potentiating its anticoagulant effect. This can lead to rapid and unpredictable increases (two to three times treatment levels) in prothrombin times. Hence it is recommended that anticoagulant doses should be reduced by one third to one half with meticulous monitoring of prothrombin times [Van Erven and Schalij, 2010]. Amiodarone is excreted in the faeces with less than 1% of the dose being excreted unchanged in the urine. The only major active metabolite of amiodarone is desethylamiodarone (DEA).
How much iodine is in amiodarone?
Amiodarone is a benzofuran compound (Figure 1) that contains approximately 37% iodine by weight and bears a remarkable structural resemblance to thyroid hormones (Figure 1(a)—(c)) [Rao et al.1986]. Therefore, a patient taking a standard 200 mg daily dose of amiodarone ingests 75 mg of organic iodine each day. Subsequent deiodination through drug metabolism results in the daily release of approximately 6 mg of free circulating iodine. This equates to a 20–40 times higher than the average daily iodine intake in the USA/UK of 0.15–0.30 mg [Basaria and Cooper, 2005; Martino et al.2001]. The oral bioavailability of amiodarone is both slow and highly variable and averages 40% [Cohen-Lehman et al.2010], ranging from 22% to 95% and is higher when taken with food [Siddoway, 2003; Latini et al.1984].
What is type 1 AIT?
Type I AIT usually occurs in patients with underlying thyroid pathology, such as latent Graves' disease or nodular goitre. In these patients, the sudden iodide load, associated with amiodarone treatment, accelerates thyroid hormone synthesis sufficiently to induce thyrotoxicosis because of increased thyroid hormone production in autonomous thyroid tissue (Jod—Basedow phenomenon) [Newman et al.1998].
What causes the release of preformed hormones?
Excessive release of preformed hormones due to thyroid destruction
What is the main substrate for thyroid hormone?
Iodine is one of the principle substrates for thyroid hormone synthesis. It is actively transported into the thyroid gland where it is incorporated into the tyrosine residues within the molecule thyroglobulin to form the precursors of both thyroxine (T4) and triiodothyronine (T3), which are enzymatically cleaved and released into the circulation [Cavalieri, 1997]. All of the steps in thyroid hormone biosynthesis, from oxidation and organification of iodide to the secretion of T4 and T3 into the circulation, are stimulated by thyroid-stimulating hormone (TSH) and inhibited by excess iodine. This autoregulation of iodine prevents hyperthyroidism in normal people who are exposed to high iodine load such as that found in radioactive contrast, an effect known as the Wolff—Chaikoff effect. Patients with underlying thyroid disease however fail to benefit from this effect and can develop hyperthyroidism [Stanbury et al.1988; Braverman et al.1971].
Why is thyroid autoregulation lost?
Normal thyroid autoregulation is lost because of the relatively high iodine content in amiodarone. This tends to occur in patients with underlying Hashimoto's disease [Braverman et al.1971]. In addition there may be iodine-related potentiation of thyroid autoimmunity and unregulated thyroid hormone synthesis in patients with underlying Graves' disease (Jod-Basedow effect).
How long does it take for iodine levels to rise after amiodarone?
TSH is the first hormone level to change, rising within 48 h and increasing up to an average of 2.7 times the normal levels by the 10th day [Basaria and Cooper, 2005]. There is also an early and measureable rise in serum T4, rT3 and free T4, which peak after 10 weeks of treatment [Melmed et al.1981]. Conversely, there is a reciprocal and early fall in serum T3 levels over a similar time course. By 3 months a steady state is generally reached with serum levels of total and free T4 and rT3 remaining at the upper end of the normal range or marginally elevated. T3 levels tend to remain at the lower end of the normal range after 3 months, with a fall in TSH to the upper end of the normal range.
What is the first line treatment for AIT2?
Conclusion: Corticosteroids are well-recognized to be the first-line treatment for AIT2. This case illustrates a rare phenomenon: successful treatment of AIT2 with lithium and cholestyramine. In patients who develop complications from first-line therapy, prompt treatment with alternative agents may successfully avert thyroidectomy and its associated risks.
What were the symptoms of thyrotoxicosis?
Apart from mild weight loss, there were no symptoms of thyrotoxicosis, neck swelling or visual symptoms. There was no prior personal or family history of thyroid disease. He was afebrile and hemodynamically stable (blood pressure 126/98 mmHg, pulse rate 83 beats per minute), which was surprising given the degree of biochemical thyrotoxicosis. This could be attributed to in-situ pacemaker with a set rate of 50 beats per minute, as well as chronic beta-blocker and amiodarone therapy. Significant findings included fine hand tremors and a small diffuse goiter, but no signs of thyroid eye disease or pretibial myxedema. There was central cyanosis, digital clubbing and a pan-systolic cardiac murmur. His pulse was regular and there were no signs of heart failure.
What is AIT1?
Amiodarone induced thyrotoxicosis (AIT) is a potentially catastrophic situation for patients with cardiac disease ( 1) who are at risk of life-threatening complications. AIT exists in two main subtypes – AIT1 and AIT2, which have different underlying pathophysiological mechanisms ( Figure 1 ). AIT1 is a form of iodine-induced hyperthyroidism caused by the high iodine content of amiodarone, while AIT2 is a form of destructive thyroiditis caused by direct cytotoxic effects of amiodarone and its metabolites.
Is carbimazole used for AIT?
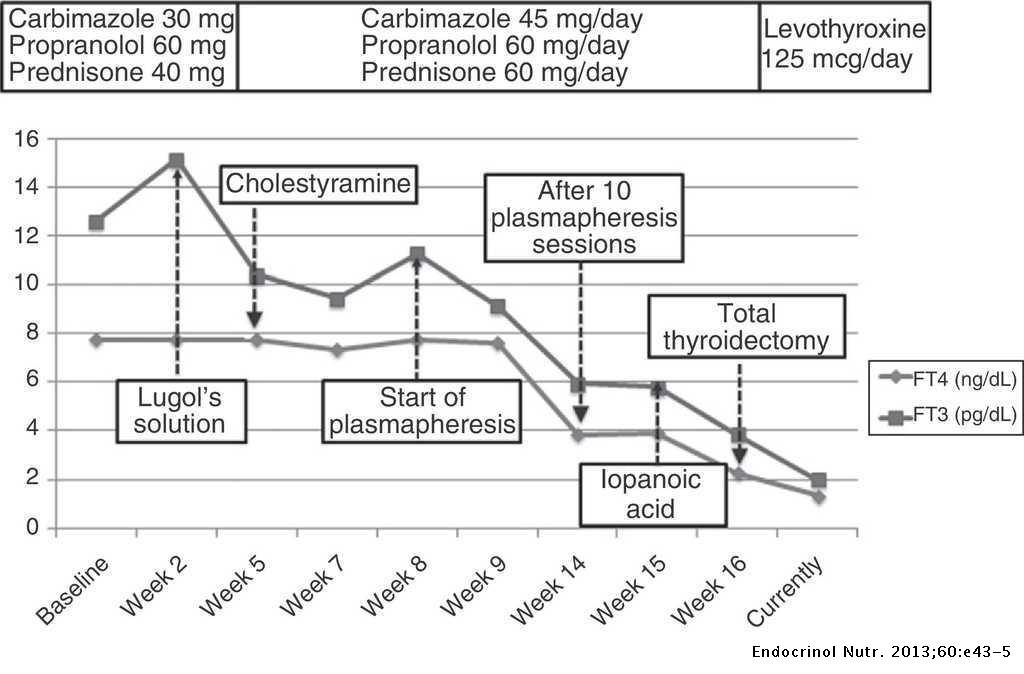
Empirical treatment for both subtypes was initiated with carbimazole 40 mg and prednisolone 40 mg daily, while awaiting further investigations to distinguish between AIT type 1 (AIT1) and type 2 (AIT2). Amiodarone was discontinued. Close liver function monitoring was ordered due to carbimazole use in the setting of hyperbilirubinemia (related to hepatic venous congestion). TSH-receptor antibodies and anti-TPO antibodies were negative, while ultrasonography showed thyroid nodules with patchy parenchymal flow. The initial report of the Technetium ( 99 Tc) pertechnetate scan described diffusely increased thyroid uptake, which suggested AIT1 ( 5, 6 ). Thus, prednisolone was stopped while carbimazole was continued. However, subsequent development of neutropenia attributed to acute viral illness necessitated carbimazole cessation. Reintroduction of prednisolone at this juncture led to significant reduction in serum thyroxine, but this also had to be discontinued within 1 week due to worsening hepatic dysfunction from acute hepatitis E infection ( Table 2 ).
Does thiamazole affect hepatic activation?
Following improvement in hyperbilirubinemia, he was started on thiamazole, which does not require hepatic activation. When he had achieved near-euthyroid status, lithium and cholestyramine were stopped ( Table 2 ). Throughout this entire period, our patient remained stable with no cardiovascular complications.
Can amiodarone be stopped?
Amiodarone is an efficient drug for life-threatening arrythmias, has a long half-life of 90 days and a cardioprotective effect ( 9, 10, 25 ). Thus, stopping amioda rone might increase the risk of life-threatening arrythmias without any short-term benefit ( 9, 25 ). Although amiodarone continuation might be feasible in AIT2 [ ( 26, 27 ), p. 2], this might lead to increased recurrence rate of thyrotoxicosis and deleterious cardiac effects ( 28 ). In our patient, as underlying atrial flutter and heart block had been treated and initial AIT subtype was unclear, we chose to discontinue amiodarone with close monitoring for recurrence of arrythmia.
Why is amiodarone thyrotoxicosis so difficult to manage?
This makes amiodarone-induced thyrotoxicosis (AIT) a difficult condition to manage, especially because data on optimal treatment are limited as the result of a lack of controlled trials.
What is AIT in a thyroid clinic?
We defined AIT as a new finding of suppressed serum TSH with raised free T 4 and free T 3 levels during amiodarone treatment. 1 All received the thionamide carbimazole (CBZ) alone (starting dose, 20 to 40 mg/d). Propylthiouracil (PTU) was prescribed if CBZ was not tolerated. Our policy of using thionamides as first-line therapy was based on our previously reported outcome in a series of 5 patients. 9 The decision about the continuation of amiodarone was made after consideration of the original indication and the availability of an alternative antiarrhythmic agent.
How long does it take to get euthyroidism from CBZ?
The median time to euthyroidism (defined as normal free T 4 and free T 3 concentrations) was 4.7 months (3 to 7 months). Eleven patients achieved sustained biochemical euthyroidism with CBZ in doses titrated according to free T 4; 5 patients (continuing amiodarone) received maintenance CBZ therapy and remained euthyroid, and 5 patients stopped CBZ after a median duration of 7.2 months (6 to 12 months) and remained euthyroid off therapy. One patient underwent total thyroidectomy 11 months after diagnosis of AIT for follicular thyroid carcinoma after being rendered euthyroid with CBZ.
How long does it take for AIT to resolve?
Five patients with AIT resolved spontaneously over a median period of 3.3 months (3.0 to 4.7 months). These patients were noted at their first clinic visit to have normal or improving thyroid function tests, despite not receiving treatment, and were observed; all achieved euthyroidism. In 4 of these patients, amiodarone was continued ( Figure ).
What is the TSH level of AIT?
The median serum free T 4 concentration at diagnosis of AIT was 48.3 pmol/L (41 to 121 pmol/L), and the mean serum free T 3 concentration was 8.2 pmol/L (7 to 35 pmol/L); TSH was undetectable (<0.1 mU/L) in all.
Is thionamide a first line treatment for AIT?
Our retrospective study suggests that treatment with a thionamide alone as first-line therapy for AIT, at least in iodine-replete areas such as the United Kingdom and United States, is appropriate, thus avoiding potential side effects of medications such as perchlorate and glucocorticoids .
Is AIT treated with methimazole?
8 The authors concluded that distinction of AIT is essential for management and suggested that type 1 be treated with both methimazole and potas sium perchlorate and type 2 be treated with glucocorticoids. This discrepancy may reflect differences in iodine intake in the 2 areas of study, with the United Kingdom being an iodine-replete area in contrast to Italy.
What is the treatment for amiodarone associated thyrotoxicosis?
Treatment of amiodarone associated thyrotoxicosis by simultaneous administration of potassium perchlorate and methimazole.
What is the best treatment for AIT type 2?
The preferred treatment of AIT type 2 according to questionnaires and clinical studies is to stop amiodarone and treat with prednisone, either alone or in combination with thionamides ( 4, 8, 9, 13 ). The rationale behind the addition of thionamides is that it is not always possible to label AIT accurately as type 2 because mixed cases of type 2 and type 1 occur ( 8, 9, 14 ). AIT type 1 is due to iodide-induced hyperthyroidism, occurring in patients with preexistent thyroid disease like nontoxic goiter or latent Graves' disease. These uncertainties have led to marked differences in proposed treatment algorithms. A United Kingdom-based algorithm recommends continuing amiodarone and starting with prednisone plus carbimazole and considering potassium perchlorate if after 2 wk T 3 levels have not decreased ( 15 ). KClO 4 is recommended in type 1 because it acutely inhibits thyroidal iodide uptake, thereby rendering the thyroid more sensitive to thionamides. An algorithm from Turkey recommends stopping amiodarone, starting with KClO 4 plus methimazole, and adding prednisone if after 1 month free T 4 (FT 4) has not normalized or decreased by more than 50% ( 16 ). Combining prednisone and KClO 4 is attractive because it may take care of mixed cases of AIT, which are believed to occur in 15–27% ( 8, 9 ). However, treatment of AIT type 2 with KClO 4 alone might also be considered ( 10 ). This strategy would avoid exposure to steroids and its related side effects, whereas KClO 4 -induced severe side effects like aplastic anemia have not been reported in AIT patients treated with lower doses of the drug (≤1000 mg/d) and for a shorter period (≤6 months) than customary in the past ( 13, 17 ). KClO 4 inhibits the cytotoxic effect of amiodarone on thyrocytes in vitro although to a lesser extent than steroids ( 3 ).
What are the criteria for AIT type 2?
AIT type 2 was ascertained if all following diagnostic criteria were met: TSH below 0.4 mU/liter and FT 4 higher than 25 pmol/liter, thyroid peroxidase antibodies below < 50 kU/liter and TSH binding inhibitory immunoglobulins below 2 U/liter, poor or no visualization of thyroid gland on 99mTc-pertechnetate scintigraphy , absence of nodular goiter (more than one nodule or a nodule > 1 cm) on ultrasonography. Exclusion criteria were no informed consent, critical illness, drug or alcohol abuse, and pregnancy.
What is AIT type 2?
Amiodarone-induced thyrotoxicosis (AIT) type 2 is caused by a destructive thyroiditis due to direct cytotoxic effects of the drug on thyrocytes ( 1 – 3 ). The nature of destructive thyroiditis is that of a self-limiting disease.
How to measure thyroid volume?
Thyroid volume was measured by ultrasonography in 14 patients. Thyroid volume was calculated using the formula width × length × thickness × 0.52 for each lobe as described previously ( 18 ). In 22 patients, it was only mentioned if the thyroid was enlarged or not. Based on this information, we divided the thyroid volumes into two categories: not enlarged (<20 ml) and enlarged (20–30 ml). The Netherlands are an iodine-replete country, and none of the patients had a thyroid volume higher than 30 ml.
Is perchlorate effective in AIT type 2?
Prednisone was the most effective treatment modality of AIT type 2. However, it is intriguing to note that perchlorate was effective in 71%. This observation raises the question of whether the success of perchlorate must be attributed to pharmacological actions of the drug ( 1, 3) or to spontaneous recovery in the natural history of AIT type 2. If one accepts the latter explanation as more likely, the next question that arises is whether prednisone really alters the natural history of the disease, i.e. shortens the time to recovery. Although many physicians would be inclined to believe so, randomized studies are lacking to substantiate this view. A recent retrospective study reported that after about 6 wk of therapy, more than 85% of patients treated with thionamides were still thyrotoxic compared with 24% of prednisone-treated patients ( 20 ). In contrast, another uncontrolled study found no difference between patients with or without prednisone treatment in terms of the time span needed to normalize FT 4 [98 (53–177) vs. 108 (64–189) d] or TSH [138 (94–220) vs. 141 (90–233) d]. The latter study did not specify AIT type and was biased by higher baseline FT 4 in the prednisone-treated patients (60 vs. 37 pmol/liter) ( 21 ).
Is prednisone used for euthyroidism?
Euthyroidism was reached despite continuation of amiodarone in all patients. Prednisone remains the preferred treatment modality of AIT type 2, because perchlorate given alone or in combination with prednisone had no better outcomes.
Case history
On June 14, 1999, a 71-yr-old man was hospitalized with a 1-week history of exertional shortness of breath, foot swelling, and feeling poorly.
Discussion
Amiodarone is an iodinated benzofuran derivative that is approved for the therapy of life-threatening recurrent ventricular arrhythmias ( 3) but is also used to treat angina, paroxysmal supraventricular tachycardia, and atrial fibrillation and to maintain normal sinus rhythm after cardioversion for atrial fibrillation ( 4 ).
Introduction
Case Presentation
- The patient was a 32-year-old gentleman with complex cyanotic congenital cardiac disease consisting of total anomalous venous drainage, double outlet right ventricle and severe pulmonary stenosis, leading to a uni-ventricular circulatory system (ejection fraction was 45% in April 2017). This was complicated by atrial flutter and complete heart block, which were treated with radiofre…
Discussion
- AIT is a diagnostic dilemma. Typical clinical features of thyrotoxicosis may be absent because amiodarone leads to decreased catecholamine receptors, decreased T3 effect on beta-adrenoceptors and inhibition of T4 to T3 conversion (9), consistent with the observation in our patient. To compound this diagnostic difficulty, management of AIT carries high stakes. AIT is a…
Conclusion
- AIT is often an insidious condition which is nonetheless important to urgently recognize and treat. In clinical practice, subtype determination is often challenging and might only be possible retrospectively, limiting the application of subtype-driven treatment. Physicians should recognize the need for urgent empirical treatment in patients with cardiac disease and establish close mo…
Data Availability Statement
- The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
- Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
- MC and SM made substantial contributions to the conception and design of this manuscript. MC and SM worked on the initial drafts and subsequently revised it critically for important intellectual content. MC and SM have viewed and are in approval of the final version of the manuscript to be published. MC and SM agree to be accountable for all aspects of the work. All authors contribut…
Conflict of Interest
- The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.