What are the disadvantages of stem cell treatments?
Feb 03, 2022 · Stem cell therapy is a form of regenerative medicine designed to repair damaged cells within the body by reducing inflammation and modulating the immune system. This phenomenon makes stem cell therapy a viable treatment option for a …
Is stem cell treatment really promising?
Mar 19, 2022 · Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply.
How much does it cost for stem cell treatment?
Stem Cell Transplants in Cancer Treatment. Stem cell transplants help restore blood-forming stem cells in people who have had theirs destroyed by certain cancer treatments. Stem cell transplants are procedures that restore blood-forming stem cells in people who have had theirs destroyed by the high doses of chemotherapy or radiation therapy that are used to treat certain …
How effective is stem cell therapy?
Stem Cell Therapy is the use of stem cells to treat or prevent a disease or condition. What are Stem Cells? Stem cells are fundamentally the building blocks of life and have the potential to differentiate into many different types of cells within the body.

What does stem cell treatment do?
Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply.
What is an example of a stem cell treatments?
The best-defined and most extensively used stem cell treatment is hematopoietic (or blood) stem cell transplantation, for example, bone marrow transplantation, to treat certain blood and immune system disorders or to rebuild the blood system after treatments for some kinds of cancer.
What diseases can be cured with stem cells?
Diseases Treated with Stem Cell TransplantsAcute leukemia.Amegakaryocytosis or congenital thrombocytopenia.Aplastic anemia or refractory anemia.Chronic lymphocytic leukemia.Familial erythrophagocytic lymphohistiocytosis.Myelodysplastic syndrome of another myelodysplastic disorder.Osteopetrosis.More items...
How long do stem cell injections last?
Stem cell treatment for knee, back, shoulder, and joint pain can have varying results in terms of how long the pain relief lasts. Several studies using stem cells as a treatment for arthritis have shown lasting results anywhere from six months to several years.Aug 30, 2018
What is the cost of stem cell therapy?
Stem cell therapy cost can range anywhere between $5000 - $50,000. Patients must do their research and ask as many questions as they can before financially committing to treatment.Dec 23, 2021
Where do you get stem cells?
Adult stem cells can be isolated from the body in different ways, depending on the tissue. Blood stem cells, for example, can be taken from a donor's bone marrow, from blood in the umbilical cord when a baby is born, or from a person's circulating blood.
Why are stem cells Bad?
Some opponents of stem cell research argue that it offends human dignity or harms or destroys human life. Proponents argue that easing suffering and disease promotes human dignity and happiness, and that destroying a blastocyst is not the same as taking a human life.Jun 14, 2007
What are the negative effects of stem cell therapy?
Stem Cell or Bone Marrow Transplant Side EffectsMouth and throat pain. ... Nausea and vomiting. ... Infection. ... Bleeding and transfusions. ... Interstitial pneumonitis and other lung problems. ... Graft-versus-host disease. ... Hepatic veno-occlusive disease (VOD) ... Graft failure.More items...•Mar 20, 2020
What are the disadvantages of stem cells?
The main disadvantage of stem cell research has to do with the way that they're acquired-that is, it involves the destruction of human embryos. This makes it immoral for those who believe that life begins at contraception.Feb 11, 2020
How painful is stem cell injections?
Injections of stem cells to most body regions are no more discomforting than any other typical joint or soft tissue injection. Injections into a spinal disc are more uncomfortable and are typically done under sedation.
Is stem cell procedure painful?
This procedure isn't painful and is done while you're awake. It takes around 3 hours and may need to be repeated the next day if not enough cells are removed the first time.
How long does it take to recover from stem cell therapy?
Recovery time depends on the type of transplant: Donated bone marrow or peripheral blood stem cell transplant can take 2-3 weeks. Cord blood engraftment can take 3-5 weeks. Self-donated stem cell transplant (autologous) takes about 10 days for recovery.
What is stem cell therapy?
Stem-cell therapy is the use of stem cells to treat or prevent a disease or condition. As of 2016. [update] , the only established therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone-marrow transplantation, but the cells can also be derived from umbilical cord blood.
Why are stem cells being studied?
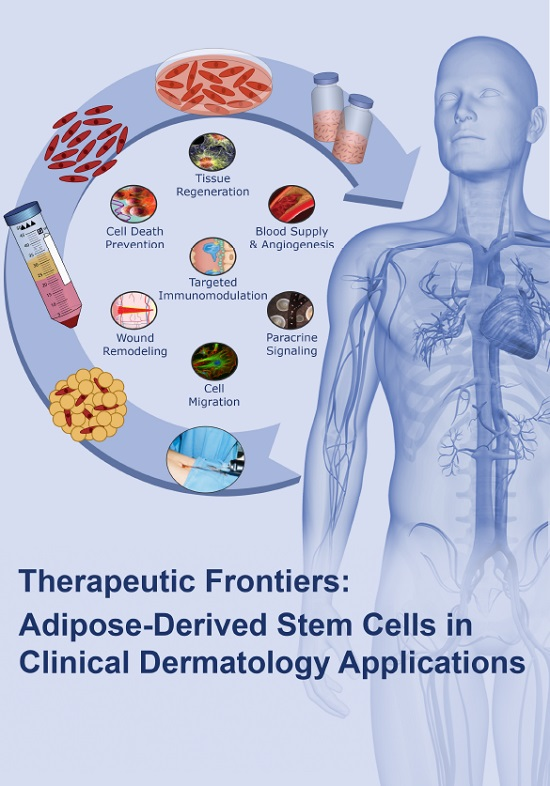
Stem cells are being studied for a number of reasons. The molecules and exosomes released from stem cells are also being studied in an effort to make medications. In addition to the functions of the cells themselves, paracrine soluble factors produced by stem cells, known as the stem cell secretome, have been found to be another mechanism by which stem cell-based therapies mediate their effects in degenerative, autoimmune, and inflammatory diseases.
How many discrepancies were found in 2013 studies of autologous bone marrow stem cells on ventricular
In 2013, studies of autologous bone-marrow stem cells on ventricular function were found to contain "hundreds" of discrepancies. Critics report that of 48 reports, just five underlying trials seemed to be used, and that in many cases whether they were randomized or merely observational accepter-versus-rejecter, was contradictory between reports of the same trial. One pair of reports of identical baseline characteristics and final results, was presented in two publications as, respectively, a 578-patient randomized trial and as a 391-subject observational study. Other reports required (impossible) negative standard deviations in subsets of people, or contained fractional subjects, negative NYHA classes. Overall, many more people were reported as having receiving stem cells in trials, than the number of stem cells processed in the hospital's laboratory during that time. A university investigation, closed in 2012 without reporting, was reopened in July 2013.
How to regenerate tissue in adult?
A possible method for tissue regeneration in adults is to place adult stem cell "seeds" inside a tissue bed "soil" in a wound bed and allow the stem cells to stimulate differentiation in the tissue bed cells. This method elicits a regenerative response more similar to fetal wound-healing than adult scar tissue formation.
What are the effects of stem cells on animal models of brain degeneration?
Research has been conducted on the effects of stem cells on animal models of brain degeneration, such as in Parkinson's disease, Amyotrophic lateral sclerosis, and Alzheimer's disease. Preliminary studies related to multiple sclerosis have been conducted.
How do corneal stem cells help restore vision?
Since 2003, researchers have successfully transplanted corneal stem cells into damaged eyes to restore vision. "Sheets of retinal cells used by the team are harvested from aborted fetuses, which some people find objectionable." When these sheets are transplanted over the damaged cornea, the stem cells stimulate renewed repair, eventually restore vision. The latest such development was in June 2005, when researchers at the Queen Victoria Hospital of Sussex, England were able to restore the sight of forty people using the same technique. The group, led by Sheraz Daya, was able to successfully use adult stem cells obtained from the patient, a relative, or even a cadaver. Further rounds of trials are ongoing.
What is the FDA approved for?
The FDA has approved five hematopoietic stem-cell products derived from umbilical-cord blood, for the treatment of blood and immunological diseases. In 2014, the European Medicines Agency recommended approval of limbal stem cells for people with severe limbal stem cell deficiency due to burns in the eye.
What is stem cell therapy?
Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply.
What are stem cells used for?
You may wonder what stem cells are, how they're being used to treat disease and injury , and why they're the subject of such vigorous debate.
How many cells are in an embryo?
Embryonic stem cells. These stem cells come from embryos that are three to five days old. At this stage, an embryo is called a blastocyst and has about 150 cells. These are pluripotent (ploo-RIP-uh-tunt) stem cells, meaning they can divide into more stem cells or can become any type of cell in the body.
What are the master cells of the body?
Stem cells are the body's master cells. All other cells arise from stem cells, including blood cells, nerve cells and others. Stem cells are the body's raw materials — cells from which all other cells with specialized functions are generated. Under the right conditions in the body or a laboratory, stem cells divide to form more cells called ...
Why are adult stem cells more likely to contain abnormalities?
Adult stem cells also are more likely to contain abnormalities due to environmental hazards, such as toxins, or from errors acquired by the cells during replication.
What are perinatal stem cells?
Perinatal stem cells. Researchers have discovered stem cells in amniotic fluid as well as umbilical cord blood. These stem cells also have the ability to change into specialized cells. Amniotic fluid fills the sac that surrounds and protects a developing fetus in the uterus.
What type of cells are used to test for drugs?
Test new drugs for safety and effectiveness. Before using investigational drugs in people, researchers can use some types of stem cells to test the drugs for safety and quality. This type of testing will most likely first have a direct impact on drug development first for cardiac toxicity testing.
What is stem cell transplant?
Stem cell transplants help restore blood-forming stem cells in people who have had theirs destroyed by certain cancer treatments. Stem cell transplants are procedures that restore blood-forming stem cells in people who have had theirs destroyed by the very high doses of chemotherapy or radiation therapy that are used to treat certain cancers.
Why are stem cells important?
Blood-forming stem cells are important because they grow into different types of blood cells. The main types of blood cells are: White blood cells, which are part of your immune system and help your body fight infection. Red blood cells, which carry oxygen throughout your body. Platelets, which help the blood clot.
What is the difference between autologous and allogeneic stem cells?
Transplants can be: Autologous, which means the stem cells come from you, the patient. Allogeneic, which means the stem cells come from someone else. The donor may be a blood relative but can also be someone who is not related. Syngeneic, which means the stem cells come from your identical twin, if you have one.
Why does graft versus tumor occur?
Graft-versus-tumor occurs when white blood cells from your donor (the graft) attack any cancer cells that remain in your body (the tumor) after high-dose treatments. This effect improves the success of the treatments.
What happens if you have graft versus host disease?
Graft-versus-host disease can occur when white blood cells from your donor (the graft) recognize cells in your body (the host) as foreign and attack them. This problem can cause damage to your skin, liver, intestines, and many other organs. It can occur a few weeks after the transplant or much later.
How long does it take for your immune system to recover from a blood transplant?
Even after your blood counts return to normal, it takes much longer for your immune system to fully recover—several months for autologous transplants and 1 to 2 years for allogeneic or syngeneic transplants.
Where do you go to get an allogeneic stem cell transplant?
When you need an allogeneic stem cell transplant, you will need to go to a hospital that has a specialized transplant center. The National Marrow Donor Program® maintains a list of transplant centers in the United States. that can help you find a transplant center.
Which stem cell treatment is the least expensive?
This is the least expensive type of stem cell treatment available because it contains the least amount of stem cells, but it is also the least effective. Amniotic fluid stem cell treatment is best used in patients with very mild degenerative conditions ...
Where are stem cells retrieved from?
The types of stem cells we use in our protocols are explained below. Umbilical cord stem cells are retrieved from the umbilical cords of healthy, live caesarean delivered births and donated by the mothers. These are by far the most effective type of stem cells that are obtained by ethic means and available today.
What is amniotic fluid?
Amniotic fluid is the fluid in which a newborn baby lives during a woman’s pregnancy.
How much does a PRP injection cost?
PRP treatments will typically range from $500-$2000 per joint. Here at Stem Cell Rejuvenation Center we provide PRP treatments with all our stem cell treatments for free of charge.
What are the receptors of stem cells?
Receptors on stem cells allow them to migrate to areas that need healing and effect change in that area, potentially becoming new cells in the area and create new tissue to help improve functionality. They secrete chemicals which activate and attract your body’s native stem cells to initial tissue regeneration.
What is HCT/P?
HUMAN CELLS AND TISSUE PRODUCTS (HCT/P) REGULATIONS. According to FDA 21 CFR 1271, an HCT/P is regulated solely under section 361 of the PHS Act and the regulations in this part if it meets all of the following criteria: (1) The HCT/P is minimally manipulated;
Does age affect stem cell treatment?
Because we use “young” stem cells, age does not affect the efficacy of the treatment. No matter your age, there is a potential for the umbilical stem cells to have a positive effect on your health. Bone marrow and adipose stem cells will have a much lower rate of success with older patients.
What Is Stem Cell Therapy?
Stem cell therapy is a revolutionary medical treatment that is showing promising results in stem cell research. It has been used for the past few decades and continues to gain popularity as a highly effective form of stem cell therapy.
What are Stem Cells?
Stem cells are naturally occurring, undifferentiated (unspecialized) cells present in multicellular organisms which can differentiate into specialized cells and divide to produce more stem cells.
How Does It Work?
Stem cell therapy works by injecting stem cells into the body of a patient. They will then migrate to damaged organs and tissues where they begin repairing them or even replace them completely with healthy tissue.
Stem Cells Help To Create New Blood Cells
Stem cells help your blood regenerate by forming new red blood cells that carry oxygen throughout your body. The therapy is a completely safe, non-surgical way to treat many conditions by giving stem cells the ability to transform into other types of tissue in order for them to help you become healthy again!
Stem Cell Therapy Can Be Used For The Treatment of Various Diseases
Stem cell therapy is used to treat a number of diseases. Curing these diseases is important because it can help with treating the root of the problem instead of just temporarily caring for symptoms.
You May Experience Pain Relief
This therapy can help you with pain relief and it’s becoming more popular as a non-invasive treatment. Stem cells can help your muscles, tendons, bones, and joints recover from pain due to different conditions such as arthritis, osteoporosis, and more.
The Treatment Helps With Faster Recovery Time
It has shown that you can recover faster if you receive stem cell therapy. This is why this treatment has become more popular over the years because it’s able to give people their life back faster than ever before.
What is genomic medicine?
Genomic medicine is bringing hope where none previously existed. This approach to cure and treat human diseases uses human biology rather than chemical compounds made in the lab to unlock techniques and therapies with the power to cure formerly incurable diseases. The use of gene therapy, CAR T cell therapy, stem cell, and other therapies is revolutionizing medicine.
Can stem cells be frozen?
One important note is that it is possible to save and freeze stem cells from the umbilical cord blood of newborn infants. Parents can elect to do this and may choose to do so because if those stem cells are needed in the future, they are an exact match to their child.
What are stem cells used for?
One primary way stem cells (in this case derived from bone marrow) are successfully used today is in helping to heal orthopedic injuries like bone fracture defects, where the bone isn’t otherwise able to heal properly, and ligament or tendon injuries. Bone marrow transplants are also used for some cancer patients.
Why are stem cells used in medicine?
Because they have the potential to develop into such a broad range of cells, stem cells can sometimes to be used to help in repair of tissue. Still, for all their inherent promise, the ways in which stem cells have been so far proven effective for medical use is far more limited.
Where do stem cells come from?
Some are human embryonic stem cells, derived from eggs fertilized in vitro (outside of the body) and donated for that purpose.
Is the stem cell industry regulated?
Why FDA Regulation Is Important. To date, the stem cell treatment industry has remained largely unregulated. The U.S. Food and Drug Administration has been working to change that, and a recent legal decision involving Florida-based U.S. Stem Cell indicates that the FDA may be able to regulate the industry, at least in part, in the future.
Does the FDA oversee stem cell research?
That includes checking to make sure any experimental treatment trial has proper oversight. “The FDA would oversee any experimental trial leading to a therapy," as well as approving stem cell treatments that are at least as safe and effective as current therapies, says Dr. Irving Weissman, director of the Stanford Institute for Stem Cell Biology and Regenerative Medicine, and a former president of the International Society for Stem Cell Research.
Does the FDA regulate stem cells?
Industry proponents say the FDA has no right to regulate stem cells, like those used by U.S. Stem Cell, which were taken from the patient's own body – in this case, their fat tissue.
Who is the chief scientific officer of stem cell?
Stem Cell and the organization’s chief scientific officer, Kristin Comella.
How much did Dawn Gusty pay for stem cell treatment?
Twice, Dawn Gusty paid $27,000 for stem cell treatments at a clinic in Tijuana, Mexico. Twice, her care there was completely out of step with accepted medical care for her multiple sclerosis. Twice, the procedure didn't work. Still, Gusty, of Kingston Springs, Tenn., isn't second-guessing herself.
What is the ISSCR?
The ISSCR advises patients to seek only stem cell treatments being tested in clinical trials approved by the FDA (or, if abroad, by a national regulatory agency such as the European Medicines Agency). It also allows for smaller studies approved by an independent Institutional Review Board (IRB) or Ethics Review Board (ERB).
Is stem cell surgery risky?
Not all unapproved stem-cell treatments involve such risky surgery. A common technique gets stem cells from fat removed via liposuction. That's less risky than brain injections but is not without risk. Byer, too, thought the early improvement he saw in his son meant the treatment was a success.
Do stem cells need FDA approval?
In the U.S., the FDA says "stem cells, like other medical products that are intended to treat, cure, or prevent disease, generally require FDA approval before they can be marketed.". Transplanting such cells, clinics argue, is a surgical procedure rather than treatment with a drug or biological product.
Is Hare all for stem cell research?
Make no mistake: Hare is all for scientific stem cell research. His concern, he says, is "hype" that glosses over an inconvenient fact: There are no new approved stem cell therapies. The danger becomes clear if you Google "stem cell treatment.".
Did Stephen Byer's son have ALS?
The unproven procedure could have killed Ben. It didn't -- but it also didn't work.
Is liposuction a benign procedure?
You still are going through a medical procedure, going to a doctor's office, being put under anesthesia, getting liposuction, and then having the material injected back into you. And the first rule doctors learn is that there is no such thing as a benign procedure," Hare says. Real Risks, Unknown Benefits.

Overview
Veterinary medicine
Research has been conducted on horses, dogs, and cats can benefit the development of stem cell treatments in veterinary medicine and can target a wide range of injuries and diseases such as myocardial infarction, stroke, tendon and ligament damage, osteoarthritis, osteochondrosis and muscular dystrophyboth in large animals, as well as humans. While investigation of cell-based therapeutics generally reflects human medical needs, the high degree of frequency and severity …
Medical uses
For over 30 years, hematopoietic stem cell transplantation (HSCT) has been used to treat people with conditions such as leukaemia and lymphoma; this is the only widely practiced form of stem-cell therapy. During chemotherapy, most growing cells are killed by the cytotoxic agents. These agents, however, cannot discriminate between the leukaemia or neoplastic cells, and the hematopoietic stem cellswithin the bone marrow. This is the side effect of conventional chemot…
Research
Stem cells are being studied for a number of reasons. The molecules and exosomes released from stem cells are also being studied in an effort to make medications. In addition to the functions of the cells themselves, paracrine soluble factors produced by stem cells, known as the stem cell secretome, have been found to be another mechanism by which stem cell-based therapies mediate their effects in degenerative, autoimmune, and inflammatory diseases.
Society and culture
In the late 1990s and early 2000s, there was an initial wave of companies and clinics offering stem cell therapy, while not substantiating health claims or having regulatory approval. By 2012, a second wave of companies and clinics had emerged, usually located in developing countries where medicine is less regulated and offering stem cell therapies on a medical tourismmodel. Like the first wave companies and clinics, they made similar strong, but unsubstantiated, claims, mai…
See also
• Autologous stem-cell transplantation
• Cardiovascular Cell Therapy Research Network (CCTRN)
• Fetal tissue implant
• Induced pluripotent stem cell
External links
• EuroStemCell: types of stem cells and their uses