What do you need to know about cancer clinical trials?
Cancer clinical trials are research studies that involve people with cancer. The goal of these studies is to find better ways to diagnose, treat and prevent cancer. The results of the trials also provide information about how to reduce the risk of cancer in people who have not been diagnosed. Clinical Trials.
How do clinical trials help cancer patients?
in a clinical research trial which may help bring further ground-breaking treatments for the disease. The clinical research team at The Clatterbridge Cancer Centre has given patient Graham Booth an injection of a therapy tailor-made to his personal DNA and ...
How to evaluate a clinical trial?
How to Evaluate a Clinical Trial Alan Franciscus Editor-in-Chief, HCV Advocate Website Observational study: a study to observe study participants to evaluate certain outcomes. For instance, an observational study may evaluate the effect of HCV treatment on quality of life, … Continue reading →
Are clinical trials a last resort treatment?
Myth: Clinical trials are a last resort when all other treatments have failed. Fact: This is a common misconception. In reality, cancer clinical trials exist for all types and stages of cancer, as well as for cancer prevention.
Can a clinical trial cure cancer?
Unfortunately, most commercially available treatments cannot cure metastatic cancer. Clinical trials offer hope and the possibility of improving outcomes for individual cancer patients, and perhaps many others. Information from studies with diverse populations is important when developing new treatments.
Are clinical trials worth it for cancer?
Each clinical trial has its own benefits and risks. But for the most part, clinical trials (other than phase 0) have some of the same potential benefits: You might help others who have the same disease by helping to advance cancer research. You could get a treatment that's not available outside of the trial.
Is clinical trial for cancer a last resort?
The benefits of participating in a clinical trial vary by person: Participants gain earlier access to new treatment. In many cases trials aren't a last resort — they may be the first choice for patients without other treatment options. Participants often don't have to pay for experimental treatment or procedures.
How long are clinical trials for cancer patients?
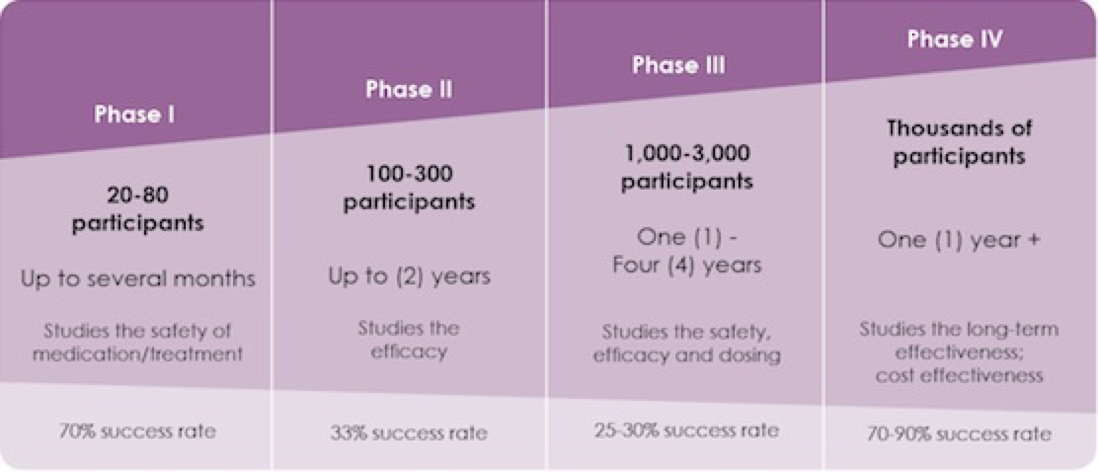
Phase I clinical trials each last several months to a year. They usually have 10 to 30 volunteers. The treatment might help the cancer. Also, information from the clinical trial may help other people in the future.
Why do cancer patients do clinical trials?
Through clinical trials, doctors determine whether new treatments are safe and effective and work better than current treatments. Clinical trials also help us find new ways to prevent and detect cancer. And they help us improve the quality of life for people during and after treatment.
Who pays for cancer clinical trials?
Every trial is different, but the clinical trial's sponsor usually pays for all research-related costs and any special testing. Typically, the patient or his or her insurance company is asked to pay for any routine tests, treatments, or procedures that would be required as part of standard cancer treatment.
Do patients get paid for clinical trials?
The answer is yes, you can get paid for study-related time and travel for participating in most clinical trials. While not all research studies pay participants, most clinical trials at Meridian pay from $75 to $4,500.
Why do clinical trials fail?
Failures can arise from a lack of efficacy, issues with safety, or a lack of funding to complete a trial, as well as other factors such as failing to maintain good manufacturing protocols, failing to follow FDA guidance, or problems with patient recruitment, enrollment, and retention.
Is clinical trial free?
There are two types of costs in a clinical trial: patient care costs and research costs. Research costs are those related to taking part in the trial. Often these costs are not covered by health insurance, but they may be covered by the trial's sponsor.
Who qualifies for clinical trials?
Each study has its own rules about who can — or cannot — participate. This is called “eligibility.” Your eligibility may be based on your age, gender, overall health, type and stage of a disease, treatment history, and other conditions. Not everyone is chosen to participate.
How do you qualify for cancer clinical trials?
Common eligibility criteria include:Having a certain type or stage of cancer.Having received (or not having received) a certain kind of therapy in the past.Having specific genetic changes in your tumor.Being in a certain age group.Medical history.Current health status.
What are the 3 stages of clinical trials?
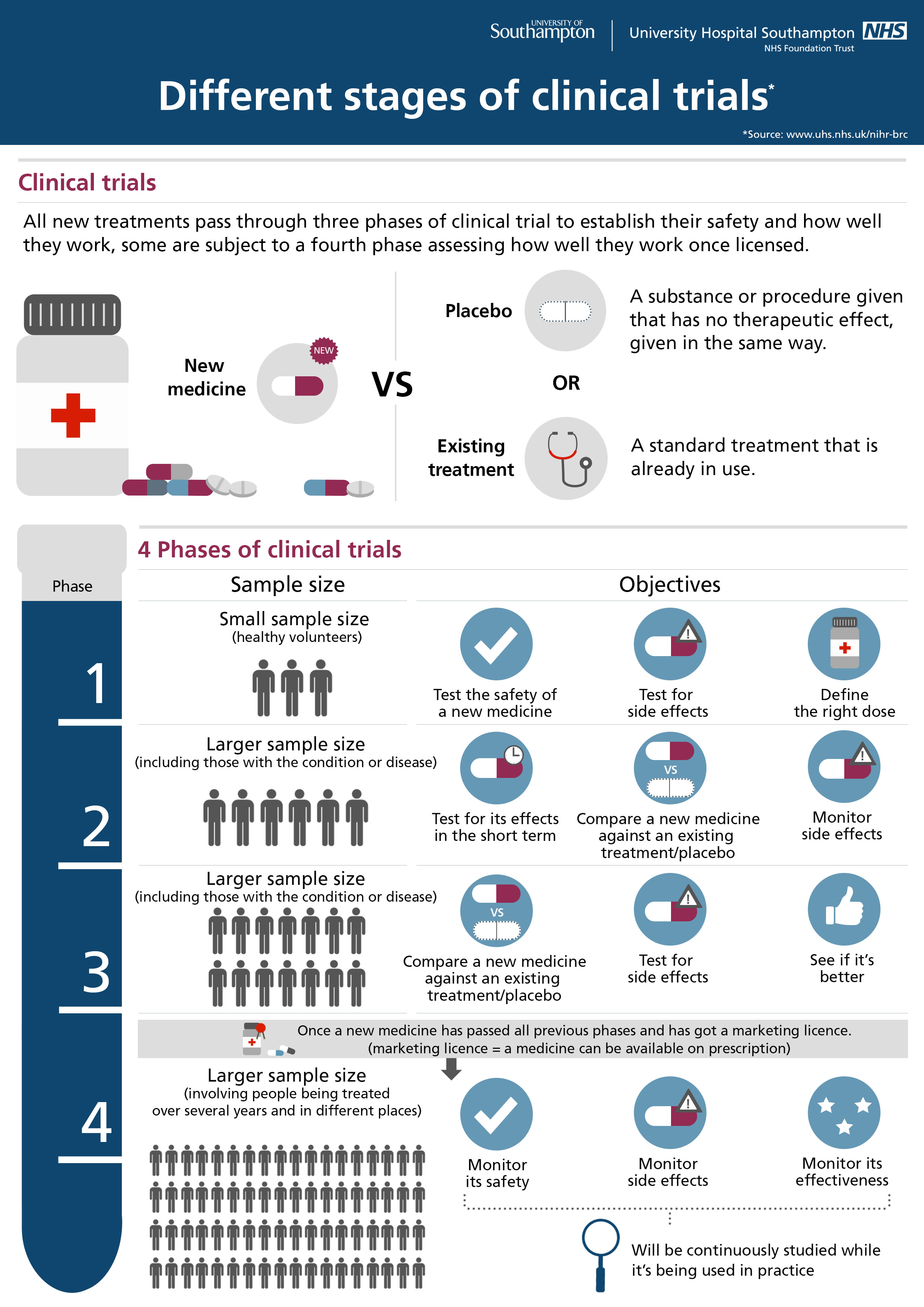
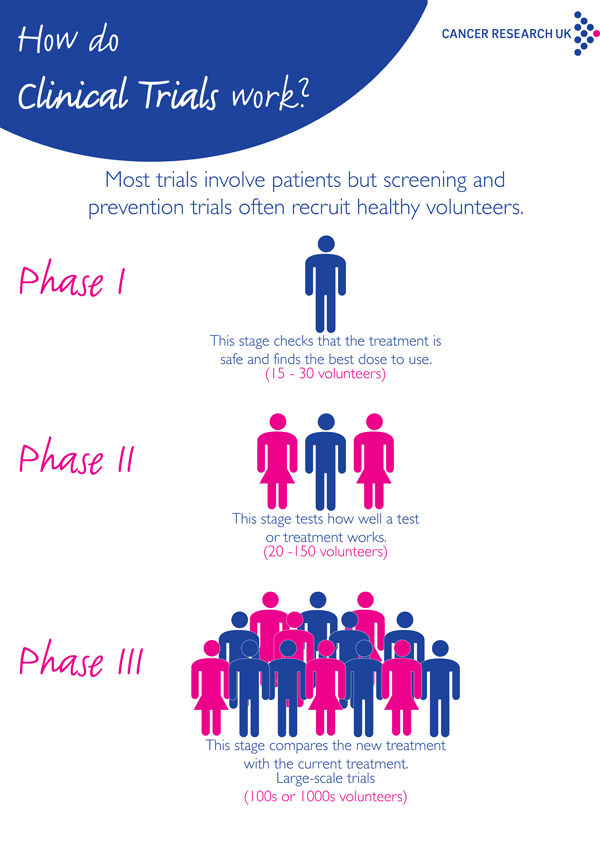
There are 3 main phases of clinical trials – phases 1 to 3. Phase 1 trials are the earliest phase trials and phase 3 are later phase trials. Some trials have an earlier stage called phase 0, and there are some phase 4 trials done after a drug has been licensed. Some trials are randomised.
Why are clinical trials important?
Clinical trials help inform our understanding of cancer and improve prevention, diagnosis, treatment, and care.
What is clinical trial?
Clinical trials are research studies that involve people. Any time you or a loved one need treatment for cancer, clinical trials are an option to think about. Learning all you can about clinical trials can help you talk with your doctor and decide what is right for you.
Do clinical trials have any benefits?
Like all treatment options, clinical trials have possible benefits and risks. Find information you can use when making your decision about whether taking part in a trial is right for you.
What is phase 3 in a drug trial?
Phase III: If the new treatment has promising activity in the phase II trial, the next step is usually a phase III trial comparing it with an existing standard treatment. If there is no standard treatment, patients may be randomly assigned to receive either the new treatment or a placebo (such as a sugar pill).
What is clinical trial?
Clinical trials are research studies that test a new drug or therapy in patients who have a disease. These studies are classified as phase I, II, or III depending on their purpose. Phase I: These initial, small studies test promising new drugs that effectively kill cancer cells in laboratory experiments. The goal is to understand the safe dose and ...
Why is phase III needed?
A phase III trial is necessary to confirm the benefits. It’s also possible that patients who come to major academic medical centers where phase I and II clinical trials are often conducted might have better outcomes than a broader population of patients.
Why are clinical trials important?
Clinical trials offer hope and the possibility of improving outcomes for individual cancer patients, and perhaps many others. Information from studies with diverse populations is important when developing new treatments.
Why do cancer trials require measurable tumors?
Some trials require patients with “measurable” tumors because shrinkage of cancer is difficult to measure without a minimum size.
What is the goal of phase 1?
The goal is to understand the safe dose and capture early evidence of benefit. Phase I trials may be open to patients with any type of cancer, or only certain types of cancers more likely to respond to specific drugs. Generally, fewer than 50 patients are enrolled. Phase II: Once a phase I trial identifies a safe dose, ...
How many patients are in a phase 2 trial?
Generally, fewer than 50 patients are enrolled. Phase II: Once a phase I trial identifies a safe dose, a phase II trial is done to better understand the potential benefit and side effects of the drug. Generally, these studies enroll fewer than 100 patients. Phase III: If the new treatment has promising activity in the phase II trial, ...
What is the protocol of a clinical trial?
The doctors and nurses who are in charge of a clinical trial follow a carefully designed treatment plan known as a protocol. The protocol spells out exactly what and why certain procedures will be done, at what intervals, and how patients will be protected by close monitoring.
What is phase IV?
A Phase IV trial might test how a newly approved drug works together with other effective drugs, or with surgery and/or radiation therapy.
How are cancer trials carried out?
Cancer clinical trials are carried out in phases: Phase I trials evaluate how a new drug should be given, how often and at what dose. After the determination of how to give the drug is complete, more patients are enrolled at that dose to get an early indication of how effective it is. A Phase I trial usually enrolls a small number ...
What is phase 2 trial?
Phase II trials test how effective a new drug or procedure is against a particular cancer. Phase III trials compare a promising new drug, new combination of drugs or new procedure with the best standard treatment. Phase III trials typically involve large numbers of patients, usually hundreds or thousands.
When is a clinical trial stopped?
If it becomes clear during a clinical trial that one treatment is better than another, the trial is stopped and all patients in the trial are offered the more effective treatment. A trial will also be stopped early if an experimental treatment is found to be ineffective or harmful.
Who approves all protocols?
No changes to the protocol are allowed to be made without consent of the FDA. All protocols must be approved by an Institutional Review Board (IRB), which consists of scientists, non-scientists, community and patient advocates.
What is phase 2 cancer?
Phase II Clinical Trials. Phase II cancer clinical trials focus on learning whether the new cancer treatments have an anti-cancer effect on a specific type of cancer. Additional information regarding the side effects of the cancer treatments is also obtained. A small number of people are included because of the risks and unknowns involved.
Why is it important to compare different groups of people taking different treatments for the same type of cancer?
Comparing similar groups of people taking different treatments for the same type of cancer is another way to make sure that the cancer clinical trial’s study results are real and caused by the treatment rather than by chance or other factors.
Why are there a small number of people in Phase III trials?
A small number of people are included because of the risks and unknowns involved. Phase III cancer clinical trials compare the results of people taking a new cancer treatment with the results of people taking a standard treatment.
What is informed consent?
Informed consent is an ongoing process during a cancer clinical trial in which all of the available information about the specific trial is discussed with the person participating in the trial. The doctor or nurse reviews the treatment plan, including potential risks and benefits of the treatment with the participant. This information is also written in a document (consent form) which is presented to the participant before treatments can begin. After the potential study participant reads the document, an opportunity to ask questions about any parts of the form that are unclear is given. If the person agrees to participate in the study, the consent form is signed. Signing the form indicates that the study participant read the form and the doctor or study nurse answered any questions about the information contained in the form, which may have been unclear. Signing a consent form does not mean a person must stay in the study. In fact, a person may leave the study at any time. If a person chooses to leave the study, a chance to discuss other treatments and care with their doctor is given, and their care will not be affected in any way.
What is cancer clinical research?
Cancer clinical trials, also called research studies, test many types of treatments such as new drugs, new surgical techniques or radiation therapy, new combinations of treatments, or new methods. The goal of the research is to find better ways to treat cancer. Cancer clinical trials include research at four different phases.
What is a phase IV trial?
Phase IV Clinical Trials. Phase IV cancer clinical trials, also called post-marketing studies, are trials conducted after a treatment has been approved.
What is a protocol in clinical trials?
The protocol is the action plan for a clinical trial. The plan states what will be done in the study and why. It outlines how many people will take part in the study, what types of patients may take part, what tests they will receive and how often, as well as the treatment plan.
Why are cancer patients not aware of immunotherapy?
Many cancer patients are not aware of immunotherapy clinical trials because their doctors do not inform them about these opportunities . We encourage patients to educate themselves about cancer clinical trials, as well as to use our Cancer Immunotherapy Clinical Trial Finder to be matched with clinical trials, and to talk with their doctors about ...
Why are clinical trials important?
Clinical trials are vital for doctors to understand how to identify and manage side effects. Some patients may believe that they will receive a ‘placebo’ (not an active drug) in place of treatment in cancer clinical trials. Patients will always receive treatment on a clinical trial if an approved treatment already exists.
Is immunotherapy available in clinical trials?
Hundreds of new and promising cancer immunotherapy treatments are only available to patients in clinical trials. Participating in clinical trials of these therapies may be the most promising option for cancer patients today, and will be critical to speeding the development and approval of new drugs for more patients in the future.
Can immunotherapy be used for cancer?
In recent years, immunotherapies have succeeded in achieving complete and durable remissions in some patients with cancers previously considered incurable. Many patients may be hesitant about participating in a cancer clinical trial for fear that the treatment may be too risky, or that the side effects may be severe.
What is a diagnostic trial?
Diagnostic trials study new ways to understand an individual person’s disease. Screening trials test the best way to detect cancer. Quality-of-life trials (also called supportive care trials) explore ways to improve comfort for people who are living with cancer. Get more in-depth information about clinical trials.
What is cancer treatment trial?
Cancer treatment trials test new drugs or new combinations of drugs. They also test new approaches to surgery or radiation therapy. Cancer prevention trials test new ways to prevent cancer in people who have never had it, or to stop it from coming back in people who have.
What is clinical trial in cancer?
For studies that involve new drugs, cancer researchers organize a clinical trial only after testing the drugs in the laboratory. The treatments that show the most promise are the ones studied in clinical trials. These are the most common kinds of clinical trials for cancer. Cancer treatment trials test new drugs or new combinations of drugs.
Why are clinical trials important?
They are an essential part of our quest to find better ways to prevent, treat, and cure cancer. Almost every cancer treatment patients receive today is the result of a clinical trial. Clinical trials are designed to answer specific questions about: There are several kinds of clinical trials for cancer. Some look at new therapies that may be able ...
Do you have to wear a mask at MSK?
Masks Are Still Required at MSK. Patients and visitors must continue to wear masks while at MSK, including people who are fully vaccinated. MSK is offering COVID-19 vaccines to all patients age 12 and over. To schedule or learn more, read this. For Adult Patients /.
How Are Clinical Trials Carried out?
- Cancer clinical trials are carried out in phases: 1. Phase I trialsevaluate how a new drug should be given, how often and at what dose. After the determination of how to give the drug is complete, more patients are enrolled at that dose to get an early indication of how effective it is. A Phase I trial usually enrolls a small number of patients but...
What About The Use of Placebos?
- In a cancer clinical trial, no patient goes without treatment if there is any known treatment that could benefit that patient. If it becomes clear during a clinical trial that one treatment is better than another, the trial is stopped and all patients in the trial are offered the more effective treatment. A trial will also be stopped early if an experimental treatment is found to be ineffectiv…
How Is Patient Safety Protected?
- The doctors and nurses who are in charge of a clinical trial follow a carefully designed treatment plan known as a protocol. The protocol spells out exactly what and why certain procedures will be done, at what intervals, and how patients will be protected by close monitoring. 1. All protocols must be reviewed and approved by the Food and Drug Administration (FDA). No changes to the …