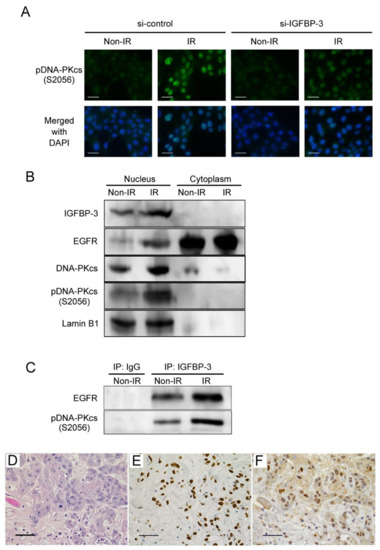
What are cancer-related biosimilars and why are they used?
They can also be used to treat side effects of cancer treatments, such as low white blood cell counts that increase the risk of infections. Below are some of the cancer-related biosimilars currently approved in the United States.
Why are biosimilar drugs becoming so popular?
The good news is that biosimilar medications are increasingly available to reduce this barrier. Each biosimilar drug is highly similar to its FDA-approved reference biologic in terms of efficacy, safety, and quality without being identical to it.
What is the difference between a biosimilar and a biologic?
In clinical trials, the biosimilar is compared to its original biologic drug, which was developed first. The original biologic is a brand name drug that has already gone through clinical trials, has been approved, and is being used to treat a disease.
Will my insurance cover a biosimilar drug?
If a biosimilar drug is a treatment option for you, it's important to talk to your insurance company about coverage. Our team is made up of doctors and oncology certified nurses with deep knowledge of cancer care as well as journalists, editors, and translators with extensive experience in medical writing.
What is the point of a biosimilar?
The primary purpose of biosimilars is to reduce the healthcare costs associated with the use of biologics and thereby increase access to healthcare. Unlike small molecule generics, the bioequivalence approach is not considered appropriate for the approval of biosimilars.
What is an example of a biosimilar?
An example of an approved biosimilar is Amjevita (adalimumab-atto), the first biosimilar approved for the blockbuster Humira (adalimumab) used to treat rheumatoid arthritis and psoriasis, among many other uses.
How does a biosimilar work?
Biosimilars basics A biosimilar is a biologic product developed to be highly similar to a previously FDA-approved biologic, known as the reference product. A biosimilar must have no clinically meaningful differences from the reference product.
What is difference between biosimilar and generic?
Generic drugs are chemically identical to the original branded drug and, as such, cost significantly less because they don't require much testing. Because biosimilars are made from living organisms, though, and don't contain identical ingredients to their name-brand counterparts, they still require some testing.
What is the difference between a biologic and a biosimilar?
Biologic drugs are large, complex proteins made from living cells through highly complex manufacturing processes. Unlike generic drugs, which are copies of chemical drugs, a biosimilar is a copy of a biologic medicine that is similar, but not identical, to the original medicine.
Which drugs are biosimilars?
FDA-Approved Biosimilar ProductsBiosimilar NameApproval DateReference ProductAmjevita (Adalimumab -atto)September 2016Humira (adalimumab)Erelzi (Etanercept-szzs)August 2016Enbrel (etanercept)Inflectra (Infliximab-dyyb)April 2016Remicade (infliximab)Zarxio (Filgrastim-sndz)March 2015Neupogen (filgrastim)33 more rows•May 26, 2022
Are biosimilars injected?
What is a biosimilar? Biologics are medicines taken by IV or self-injection and are made up of large protein molecules derived from living cells.
What diseases do biosimilars treat?
Biosimilars are safe and effective medications for treating many illnesses such as chronic skin and bowel diseases (like psoriasis, irritable bowel syndrome, Crohn's disease and colitis), arthritis, kidney conditions, and cancer.
What are the benefits of biosimilars?
Biosimilars may offer a number of potential benefits to various stakeholdersIncreased use. of biologics. Additional treatment choices at lower cost to the health care system.Improved access. and outcomes. ... Health care system. efficiency. ... Expanded options. for patients.
Why are biosimilars cheaper?
Biosimilars cost less because the path to their approval is shorter and cheaper. Manufacturers do not need to go through the same number of clinical trials and spend as much on research and development as biologics.
How are biosimilars approved?
The FDA approves a biosimilar after a manufacturer establishes that the product is highly similar to a previously approved originator biologic reference product without any clinically meaningful differences in safety, purity, and potency.
Are biosimilar drugs FDA approved?
Biosimilars Are Safe and Effective The FDA's thorough evaluation ensures that all biosimilar products are as safe and effective as their reference products and meet the FDA's high standards for approval.
What kind of cancer is biosimilar used for?
What kinds of biosimilars are used to treat cancer? In the United States, FDA-approved biosimilars can be used to treat breast cancer, stomach cancer, colorectal cancer, and other cancers. They can also be used to treat side effects of cancer treatments, such as low white blood cell counts that increase the risk of infections.
What is a biosimilar?
A biosimilar is a version of a known and proven biologic drug. Biologic drugs are drugs that are made from living things like cells, tissues, or proteins. Vaccines and antibodies are examples of biologic drugs. Biologic drugs are important in cancer treatment for several reasons.
Why are biosimilars not considered generic?
Biosimilars are not considered generic because there may be small differences between the biosimilar and its reference drug. However, these variations do not cause clinical differences in the biosimilar’s safety or how well it works. 2.
What is the FDA approved drug for cancer?
From 2018 to 2019, the FDA approved pegfilgrastim-jmdb (Fulphila), pegfilgrastim-cbqv (Udenyca), and pegfilgrastim-bmez (Ziextenzo), which are biosimilars that help fight infection, specifically in people with non-myeloid cancer treated with chemotherapy. Their reference drug is pegfilgrastim (Neulasta). In November 2018, the FDA approved ...
Why is a biologic considered a reference drug?
The biologic drug is often called a “reference drug” because the biosimilar is based on the approved drug. The biosimilar works the same way as its reference drug and also has to be approved by the FDA. A biosimilar is somewhat like a generic version of a biologic drug, though the 2 are not entirely the same.
What is the drug for non-Hodgkin lymphoma?
In November 2018, the FDA approved rituximab-abbs (Truxima) as the first biosimilar to treat people with non-Hodgkin lymphoma. Its reference drug is rituximab (Rituxan). Rituximab-pvvr (Ruxience) is another FDA-approved biosimilar to rituximab.
Why are biologic drugs so expensive?
Biologic drugs are expensive. They treat some of the most difficult, deadly diseases. They are also more complex in structure than other drugs. They are often tricky to manufacture and store because they are made from living things.
Why are biosimilars important?
Biosimilars have potential advantages in the treatment of cancer, as they introduce competition into the drug development process, which can lead to cost savings for patients and spur the development of new treatments.
What is the FDA's approach to biosimilars?
A “totality of the evidence” approach is taken by the FDA in evaluating biosimilars for approval. The results from clinical trials are important, but data from “preclinical” work is also considered.
How many biosimilars are there for filgrastim?
Including filgrastim-sndz, there are currently twelve biosimilars approved by the FDA for the treatment of specific conditions. In 2016 and 2017, the FDA approved six immunosuppresant drugs for the treatment of certain forms of arthritis, psoriasis, ulcerative colitis and Crohn’s disease.
What is bioequivalence in medicine?
A. Bioequivalence is the primary factor in the FDA’s approval of generic (chemical-based) drugs. It means that the generic drug delivers the same amount of active ingredient (s) to the targeted cancer cells as does the original brand-name drug.
What is the purpose of the BPCI Act?
The BPCI Act created an abbreviated approval process for biosimilars, the goal of which is to demonstrate “biosimilarity” between the proposed product and the original product , not to independently establish the safety and effectiveness of the proposed product.
Why are clinical trials important?
For this reason, doctors and researchers urge people with cancer to take part in clinical trials.
When does the FDA approve ogivri?
In December 2017, the FDA approved trastuzumab-dkst (Ogivri) for the treatment of people with HER2-positive breast cancer or HER2-positive gastric cancer. HER2 is a growth-promoting protein found on the surface of some cancer cells. The original drug is trastuzumab (Herceptin); its patent expires in 2019. 2018.
What are biosimilars for cancer?
Biosimilar Medications Approved for Cancer Patients 1 Mvasi (bevacizumab-awwb) Biosimilar to Avastin (bevacizumab) for the treatment of multiple types of cancer. learn move 2 Zarxio (filgrastim-sndz) Biosimilar to Neupogen (granulocyte colony stimulating factor) for treatment of chemotherapy induced neutropenia. 3 Granix (tbo-neulasta) Biosimilar to Neulasta (filgrastim) for treatment of chemotherapy induced neutropenia. 4 Ziextenzo (pegfilgrastim-bmez) Biosimilar to Neulasta (filgrastim) for treatment of chemotherapy induced neutropenia. 5 Truxima (rituximab-abbs) Biosimilar to Rituxan (rituximab) for patients with CD20-positive, B-cell non-Hodgkin’s lymphoma to be used as a single agent or in combination with chemotherapy. 6 **Ruxience (**rituximab-pvvr) Biosimilar to Rituxan (rituximab)for patients with CD20-positive, B-cell non-Hodgkin’s lymphoma or CLL. 7 Herzuma (trastuzumab-pkrb) Biosimilar to Herceptin (trastuzumab) for the treatment of HER2-positive breast cancer. 8 Kanjinti™ (trastuzumab-anns) Biosimilar To Herceptin (trastuzumab) for treatment of HER2-positive gastric and breast cancer. 9 Ogivri (trastuzumab-dkst) Biosimilar to Herceptin (trastuzumab) for the treatment of patients with HER2-overexpressing breast or metastatic gastric or gastroesophageal junction adenocarcinoma. 10 Zirabev (bevacizumab-bvzr) Biosimilar to Avastin (bevacizumab)for the treatment of patients with metastatic colorectal cancer; unresectable, locally advanced, recurrent or metastatic nonsquamous non–small-cell lung cancer (NSCLC); recurrent glioblastoma; metastatic renal cell carcinoma; and persistent, recurrent or metastatic cervical cancer.
How much savings did Norway have with biosimilars?
When biosimilars were allowed in Norway, a 60% savings were gained. It is unclear what the savings will be in the United States and whether or not those savings will be passed on to the patients remains to be determined. Many questions remain regarding the use of biosimilars in the United States.
How much did biologics cost in 2016?
The prices of the originator biologics have doubled, at a minimum since their release in 1998. The retail cost is between thirty to fifty thousand dollars annually. When biosimilars were allowed in Norway, a 60% savings were gained.
What is interchangeability in pharmacy?
Interchangeability would allow the insurance plan or specialty pharmacy to substitute the biosimilar for the originator biologic without making the prescribing physician aware that the change had occurred. The requirements for interchangeability are higher than those to achieve biosimilar status.
How long does a pharmaceutical company have to patent a drug?
A pharmaceutical company has 20 years of patent protection on a drug from the time the drug is invented in the research phase. Once the patent-protection ends, competitor companies can produce a copy of that small molecule drug, which is considered a generic form of the drug. A generic form of the drug can easily be made because the structure ...
What are biologics made of?
Biologics consist of a number of protein strands that take the form of antibodies and are derived from genetically engineered living cells, usually bacteria. 1 Biologics have a sequence of amino acids that form a specific shape that give that protein its ability to decrease disease activity.
Why is it so difficult to create a generic for biologics?
Creating a generic drug for biologics like anti-TNF drugs is much more complicated because their structure and mixture of molecules is more difficult to identify or characterize compared to small molecule conventional drugs.
What is biosimilars?
Biological products, including biosimilars, are large and generally complex molecules. Credit: Food and Drug Administration. When the patent on the cancer drug trastuzumab (Herceptin) expires next year, patients who have been receiving this biological therapy will have another treatment option: a biosimilar drug —a drug that is very similar, ...
How to establish biosimilarity?
Lim explained, is to characterize the chemical structure and biological function of the proposed biosimilar in a comparative fashion to the reference product. “The thinking is that if a biosimilar has a highly similar structure and function as the reference product, ...
What is extrapolation in biosimilars?
Extrapolation is a familiar concept among regulators, Dr. Hurvitz noted. “Clinicians are going to have to be trained in the concept of extrapolation if they are to embrace the approval of biosimilars for applications beyond the initial approved setting.”.
What is a biosimilar to Neupogen?
Five of the eleven biosimilars approved by FDA since then are for patients with cancer. The first biosimilar to be approved in the United States, in 2015, was filgrastim-sndz (Zarxio), a biosimilar to filgrastim (Neupogen), which is used to prevent infection during chemotherapy. FDA has since approved ten other biosimilar products, ...
How many biosimilars have been approved in Europe?
Thirty biosimilars have been approved in Europe since 2006, and these agents have helped to “lower costs and increase patient access to biologics,” the President’s Cancer Panel noted in its report. The report highlighted a study on the use of biosimilars in Europe. Exit Disclaimer.
How much did Medicare spend on biosimilars in 2005?
In 2005, biological products made up 39.1% of the $9.5 billion in Medicare drug spending. By 2014, these agents accounted for 62% of the $18.5 billion spent by Medicare on prescription drugs. In a recent report on the rising costs of cancer drugs, the President’s Cancer Panel concluded that biosimilars may play a role in reducing these expenses.
When was biosimilarity approved?
The Concept of Biosimilarity. Congress paved the way for the approval of biosimilars in 2010 with the passage of the Biologics Price Competition and Innovation Act, which created an abbreviated regulatory process for biosimilars. Five of the eleven biosimilars approved by FDA since then are for patients with cancer.
What is the role of biosimilars in cancer treatment?
Biosimilars and Their Role in Cancer Treatment. When chemical-based drugs are approved by the United States Food and Drug Administration (FDA), the company that developed the drug is given a patent—the exclusive right to produce and market the specific drug for a set number of years. After the patent expires, other companies are allowed ...
Why are biosimilars important?
Biosimilars have potential advantages in the treatment of cancer, as they introduce competition into the drug development process, which can lead to cost savings for patients and spur the development of new treatments.
What is cancer care social worker?
Cancer Care ’s oncology social workers are licensed professionals who counsel people affected by cancer, providing emotional support and helping people access practical assistance. Call 800-813-HOPE (4673) or visit www.cancercare.org for more information.
What is the name of the biosimilar drug that helps the body make white blood cells?
The first biosimilar, filgrastim-sndz (Zarxio), was approved by the FDA in 2015. Both filgrastim-sndz and the original product, filgrastim (Neupogen) are bone marrow stimulants, which can help the body make white blood cells after cancer treatments. Administered through an injection, filgrastim-sndz is the only biosimilar currently approved by ...
What are biologics used for?
Monoclonal antibodies are an especially important biologic; they are used in the treatment of many conditions, including breast cancer, lymphoma, rheumatoid arthritis, psoriasis, ulcerative colitis, ...
Is filgrastim sndz biosimilar?
Administered through an injection, filgrastim-sndz is the only biosimilar currently approved by the FDA for the treatment of cancer patients . The Role of Pharmacists in Your Cancer Treatment Journey. Pharmacists are highly-accessible members of the health care community.
Do biologics have to be approved by the FDA?
Biologics are also approved by the FDA and given a patent, and other companies are allowed to compete once that patent expires. However, those competing products have allowable differences because they are made from a living organism, and are called “biosimilars.”.

Are Biosimilars The Same as Generic Drugs?
- You've probably heard about generic drugs. A generic drug is an exact copy of a brand name drug. Generic drugs work the same way and can be used in the same ways as their brand name drugs. In other words, a generic drug is an equal substitute for its brand name drug and can be used to …
Are Biosimilars Safe?
- Like other medicines, a biosimilar needs to be tested in clinical trials and approved by the FDA before it can be used to treat a disease. In clinical trials, the biosimilar is compared to its original biologic, which was developed first. The original biologic is a brand name drugthat has already gone through clinical trials, has been approved, and is being used to treat a disease. Clinical trial…
Why Are Biosimilars Being developed?
- Biologics are often very expensive because they cost a lot to study and make. And unlike the case with drugs, where generic versions can lead to increased competition and lower prices, until fairly recently there were no alternate versions of biologics. The high cost of biologics can sometimes make it hard for a person to get them, even if they might be the best treatment for a disease. To …
Are Biosimilars Covered by Insurance?
- Some insurance companies will cover the cost, or part of the cost, of a biosimilar. Others might not. If a biosimilar is a treatment option for you, it's important to talk to your insurance company about coverage.