What is the efficacy of Viekira Pak for hepatitis C?
In clinical studies Viekira Pak, with or without ribavirin ( Rebetol) HCV was cured in 95% to 100% of patients treated. Cure was defined as undetectable levels of HCV in the blood 3 months after treatment. The FDA approved Viekira Pak in December 2014.
What is the viral load for hepatitis C?
The viral load for hepatitis C refers to the amount of hepatitis C virus (HCV) present in a person’s bloodstream. A hepatitis C viral load can indicate if people have contracted an active hepatitis C infection or whether treatment is reducing levels of the virus.
What should I know about Viekira Pak before taking it?
Viekira Pak can be used in people who have compensated cirrhosis. Viekira Pak is not for people with advanced cirrhosis (decompensated). If you have cirrhosis, talk to your healthcare provider before taking Viekira Pak.
How is Hep C treated if my viral load is low?
Usually, your hep C treatment will be the same no matter how high or low your viral load is. Your doctor will use your virus levels to monitor how you respond to interferon, interferon plus ribavirin, or other drugs.
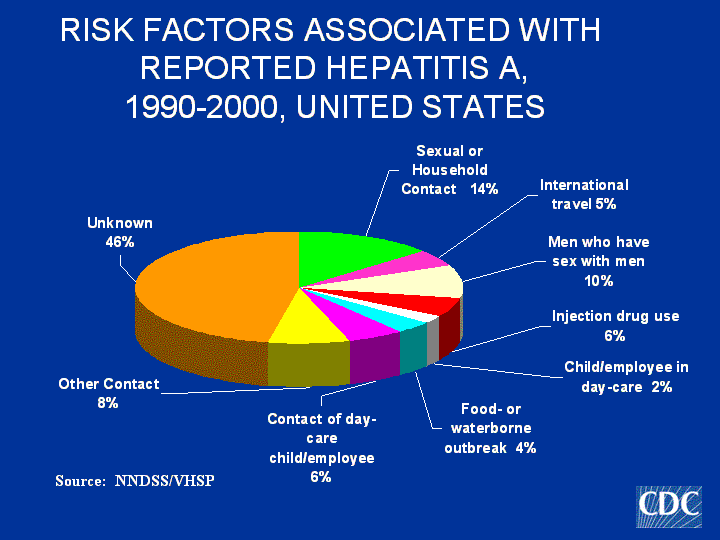
Does hep C viral load fluctuate?
Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection.
What is a high hep C viral load?
High viral load: This is when your count is more than 800,000 IU/mL. If your viral count is high at the start, it can be hard or impossible for your treatment to completely get rid of the virus. Some researchers consider high levels anything above 400,000 IU/mL. Low viral load: This is a count below 800,000 IU/mL.
Can hep C come back after interferon treatment?
Combination therapy of interferon and ribavirin has traditionally been used to eradicate hepatitis C virus. The sustained virologic response achieved with interferon-related therapy is persistent, and late relapses after achieving sustained virologic response at 24 weeks using this therapy are reportedly rare (< 1%).
Can HCV spread after treatment?
There is no virus to spread. The person is not contagious. Remember that after cure or after the body clears hep C on its own during the acute ( new) phase of infection, we can catch hepatitis C again. If this occurs, there is a new infection and yes this can be spread.
When do you treat hep C viral load?
For treatment-naïve noncirrhotic patients, with chronic HCV and a viral load below 6 million international units/mL (IU/mL), treatment duration is generally 8 weeks. However, with a viral load above 6 million IU/mL or with cirrhosis, ledispasvir/sofosbuvir is given for 12 weeks.
What is a high viral load?
Viral load refers to the amount of virus that can be detected in an infected person. High viral loads are concerning because they can mean the person is more infectious.
What are the chances of hep C returning after treatment?
The good news is that HCV is more treatable now than ever before, which explains its high cure rate. In fact, once you're considered cured, the average risk of recurrence is less than one percent. Although treatments are better, it's still possible to contract a new infection in the future.
How can hep C come back after treatment?
Relapses usually occur in the first few months after blood testing to confirm that the virus is no longer detectable. Sometimes, however, a relapse becomes evident much later. Although the exact cause of relapse is unknown, the remote possibility that the infection may return is further reason not to drink.
Can you do hep C treatment twice?
Having had hep C once does not make you immune from getting it again. You can be reinfected with hep C whether you clear the virus by successful treatment (called a sustained virologic response, or SVR) or by spontaneously clearing it on your own.
What happens if hep C treatment doesn't work?
Once someone is resistant to a certain direct-acting antiviral drug, it may not work if they try it again in the future. Cirrhosis. Over the years, hepatitis C inflammation causes permanent damage and scarring of the liver, called cirrhosis. Treatment failure rates are 15 to 20 percent higher in people with cirrhosis.
Can liver regenerate after hep C cure?
Here's an amazing fact: Once you're cured of Hepatitis C, liver damage stops. And over time (different for everyone, but possibly five years or more), your liver can heal itself through regeneration. That's right, the thing grows back!
What is the success rate of hep C treatment?
Hepatitis C treatment can cure more than 90 percent of hepatitis C cases, but testing is a critical first step. It's estimated 40 percent of people with hepatitis C in the U.S. from 2015-2018 were unaware of their infection.
How long does it take for hep C to cure?
The goal of hep C therapy is to drop your virus count low enough so it’s undetectable. If that’s the case 3 months after you finish your treatment, you’re considered cured. This happens in more than 90% of people who get the recommended treatments.
What does it mean when you have hep C?
It means your disease is in remission and your hep C is no longer active. Your liver may start to heal, and your chances for liver failure and liver cancer may drop. To confirm, you may need to repeat the test or take a qualitative test that checks if you’re negative for any trace of viral genetic material.
What is viral load?
Your viral load is how much hepatitis C virus (HCV) is in your blood. Your starting level can give a clue to your chances of success with treatment. Changes in your viral load also can tell your doctor if you’re sticking to your therapy and if you’re getting enough drugs to control your disease. But your viral load measures only what’s happening in ...
What does it mean when you have a viral load test?
Viral Load Tests. They check your blood for HCV’s genetic footprints. If any are found, it means that you have active hep C and that your viruses are multiplying. Viral load tests come in two types: Qualitative: This can confirm if you have hep C or not. A positive test means it found HCV genetic code in your blood.
What does it mean when a test is positive for HCV?
A positive test means it found HCV genetic code in your blood. Negative means it found no measurable virus. Qualitative tests are very sensitive, meaning that if you have a current hep C infection, they almost always will find it. Quantitative: This is often called a hep C RNA test.
Does hep C have the same effect on viral load?
Usually, your hep C treatment will be the same no matter how high or low your viral load is. Your doctor will use your virus levels to monitor how you respond to the medication. The drugs you’re prescribed will depend less on your viral count than on your overall health, genetic makeup of your HCV, and other things.
What are the side effects of Viekira Pak?
Common side effects include nausea, itching, and sleep problems.
What is Viekira Pak used for?
Viekira Pak and Technivie are used to treat chronic hepatitis C, a viral infection that can last a lifetime and lead to serious liver ...
Can Viekira Pak cause liver damage?
Food and Drug Administration (FDA) is warning that hepatitis C treatments Viekira Pak and Technivie can cause serious liver injury mostly in patients with underlying advanced liver disease. As a result, we are requiring the manufacturer to add new information about this safety risk to the drug labels.
When should hepatic testing be performed?
Hepatic laboratory testing should be performed at baseline, during the first 4 weeks of starting treatment , and as clinically indicated. If alanine aminotransferase (ALT),bilirubin, or both are elevated above baseline levels, repeat the test and monitor closely.
Is Viekira Pak contraindicated?
FDA emphasizes that Viekira Pak and Technivie are contraindicated in moderate and severe hepatic impairment (Child-Pugh Class B & C). Technivie is not indicated for use in patients with cirrhosis. Some of the postmarketing cases of hepatic failure occurred in patients for whom Viekira Pak and Technivie are contraindicated or not recommended.
How long does Viekira pak last?
Patients with HCV genotype 1a with cirrhosis may be treated with Viekira Pak plus ribavirin for 24 weeks. (Some patients who have had prior treatment with other drugs may be considered for a shorter treatment duration of 12 weeks.) Patients with HCV genotype 1b without cirrhosis may be treated with Viekira Pak for 12 weeks.
What is Viekira Pak?
Viekira Pak (ombitasvir, paritaprevir, ritonavir, and dasabuvir) is a combination drug prescribed to treat hepatitis C infection to include those patients with compensated liver cirrhosis. Side effects, drug interactions, dosing, storage, and pregnancy and breastfeeding information should be reviewed prior to taking this medication.
How many tablets are in Viekira?
The recommended dose of Viekira Pak is two tablets containing ombitasvir, paritaprevir, ritonavir in the morning and one dasabuvir tablet in the morning and evening. Viekira Pak should be taken with meals.
What is the difference between ombitasvir and paritaprevir?
They block the effect of proteases which are enzymes that HCV uses for making new virus, leading to reduced numbers of HCV copies in the body. Ombitasvir blocks HCV NS5A protease while paritaprevir blocks HCV NS3/4A protease.
How many drugs are in Viekira Pak?
It contains four medicines, dasabuvir, ombitasvir, paritaprevir, and ritonavir. Viekira Pak belongs to a class of drugs called direct-acting antiviral agents. Similar drugs include. ombitasvir, paritaprevir, ritonavir (Technivie). Dasabuvir, ombitasvir, and paritaprevir are antivirals that work in three different ways to help stop HCV ...
What medications can I take to treat Viekira Pak?
Certain anti- seizure medications, including carbamazepine ( Tegretol ), phenytoin ( Dilantin ), and phenobarbital (Luminal), may decrease the blood concentrations of Viekira Pak and reduce the effectiveness of treatment.
What is the name of the antiviral that works in three different ways?
sofosbuvir ( Sovaldi ), simeprevir ( Olysio ), telaprevir ( Incivek ), and. ombitasvir, paritaprevir, ritonavir (Technivie). Dasabuvir, ombitasvir, and paritaprevir are antivirals that work in three different ways to help stop HCV from multiplying. They block the effect of proteases which are enzymes that HCV uses for making new virus, ...
What is hepatitiscentral.com?
HepatitisCentral.com provides information regarding hepatitis and liver disease. Comments are available to the community in order to discuss these topics and obtain answers to questions through community members. The Editors at HepatitisCentral.com will not be responding to questions or comments posed in article comments.
Is hepatitis C cured?
There is much debate on whether or not successful Hepatitis C treatment is equivalent to a cure; however, attaining sustained virologic response (SVR) is considered to be the closest you can get to being cured of this virus. SVR is defined as having no detectable Hepatitis C in the blood six months following the end of therapy. In trials, the Viekira Pak has demonstrated a high cure rate.
What percentage of subjects with Viekira Pak with RBV had a dose adjustment?
Seven percent of subjects (101/1551) treated with Viekira Pak with RBV had a RBV dose adjustment due to a decrease in hemoglobin level; of these, 98% (98/100) achieved an SVR12.
What are the side effects of Viekira Pak?
Adverse events occurring in more than 20% of subjects included fatigue 50%, headache 44%, cough 32%, diarrhea 26%, insomnia 26%, asthenia 24%, nausea 24%, muscle spasms 21% and rash 21%. Ten subjects (29%) had at least one post-baseline hemoglobin value of less than 10 g/dL. Ten subjects underwent a ribavirin dose modification due to decrease in hemoglobin and 3% (1/34) had an interruption of ribavirin. Five subjects received erythropoietin, all of whom initiated ribavirin at the starting dose of 1000 to 1200 mg daily. No subject received a blood transfusion [see Clinical Studies ( 14.5 )].
What enzymes are involved in the metabolism of Paritaprevir?
Paritaprevir and ritonavir are primarily metabolized by CYP3A enzymes. Co-administration of Viekira Pak with strong inhibitors of CYP3A may increase paritaprevir and ritonavir concentrations. Dasabuvir is primarily metabolized by CYP2C8 enzymes. Co-administration of Viekira Pak with drugs that inhibit CYP2C8 may increase dasabuvir plasma concentrations. Ombitasvir is primarily metabolized via amide hydrolysis while CYP enzymes play a minor role in its metabolism. Ombitasvir, paritaprevir, dasabuvir and ritonavir are substrates of P-gp. Ombitasvir, paritaprevir and dasabuvir are substrates of BCRP. Paritaprevir is a substrate of OATP1B1 and OATP1B3. Inhibition of P-gp, BCRP, OATP1B1 or OATP1B3 may increase the plasma concentrations of the various components of Viekira Pak.
How long does Viekira Pak last?
Viekira Pak with RBV was administered for 24 weeks to 34 HCV GT1-infected liver transplant recipients who were at least 12 months post transplantation at enrollment with normal hepatic function and mild fibrosis (Metavir fibrosis score F2 or lower). The initial dose of RBV was left to the discretion of the investigator with 600 to 800 mg per day being the most frequently selected dose range at initiation of Viekira Pak and at the end of treatment.
What is Viekira Pak?
Viekira Pak is indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) [see Dosage and Administration ( 2.2) and Clinical Studies ( 14 )]: genotype 1b without cirrhosis or with compensated cirrhosis.
Is Viekira Pak in human milk?
It is not known whether Viekira Pak and its metabolites are present in human breast milk, affect human milk production or have effects on the breastfed infant. Unchanged ombitasvir, paritaprevir and its hydrolysis product M13, and dasabuvir were the predominant components observed in the milk of lactating rats, without effect on nursing pups [see Data].
Is Viekira Pak contraindicated?
If Viekira Pak is administered with ribavirin, the combination regimen is contraindicated in pregnant women and in men whose female partners are pregnant. Refer to the ribavirin prescribing information for more information on use in pregnancy.