What is heat treatment of martensitic stainless steel?
Heat treatment of Martensitic stainless steel is essential to achieve improve strength, fracture toughness, and hardness depending on the carbon content. Martensitic stainless steels are sensitive to heat treatment variables. Before any heat treatment, prior cleaning is required to avoid contamination from oil, grease and any source of carbon.
What happens when martensite is heated?
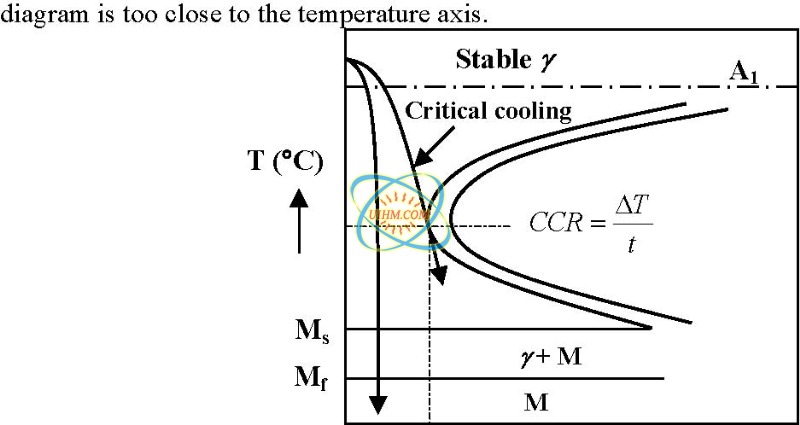
Jun 01, 2011 · In order to form martensite we need to heat steel into the austenite field (above Ac 3) and quench rapidly enough from the austenite phase to avoid pearlite formation. The rate must be fast enough to avoid the nose of the Time-Temperature-Transformation (TTT) curve – the so-called critical cooling rate for the given steel.
What are the different ways of forming martensite?
Jan 28, 2009 · Tempering consists of reheating the martensite to temperatures typically between 275-750°F (135–400°C) for several hours. During the temper heat treatment, carbides precipitate in the martensite matrix. This transformation increases the toughness of the steel by the carbide precipitates as well decreasing the carbon content of the martensite.
What is a martensite transformation when cooled quickly?
The resulting martensitic steel is extremely hard but very brittle. Thus, the martensite is then heated in a process called tempering, which causes the martensite to transform partially into ferrite and cementite. Figure 8 - Surface markings due to the formation of martensite plates in Fe-Ni single crystals.

What is martensite in heat treatment?
How martensite is produced?
Which one will produce martensite phase?
Which heat treatment effect is associated with coarse martensite?
How do you make tempered martensite?
At what temperature is martensite heated in tempering?
Which heat treatment increases the toughness of high carbon steel?
At what temperature does martensite form?
How can martensite be prevented?
Which heat treatment process is carried out for converting retained austenite into martensite?
What is the name of heat treatment process done to relieve strain and stress?
What are the various types of heat treatment?
- Heat Treatment Steel: Annealing.
- Heat Treatment Steel: Normalizing.
- Heat Treatment Steel: Hardening.
- Heat Treatment Steel: Tempering.
Is martensite tempered?
All steels containing martensite should be tempered. As heat treaters, we need to know that martensite in steel produces a hard, brittle microstructure that must be tempered to provide the delicate balance necessary between strength and toughness needed to produce a useful engineering material.
Who is Dan Herring?
Dan Herring is president of THE HERRING GROUP Inc. , which specializes in consulting services (heat treatment and metallurgy) and technical services (industrial education/training and process/equipment assistance). He is also a research associate professor at the Illinois Institute of Technology/Thermal Processing Technology Center. tel: 630-834-3017; e-mail: [email protected]; web: www.heat-treat-doctor.com
What is martensite made of?
Martensite is a hard, brittle form of steel with a tetragonal crystalline structure , created by a process called martensitic transformation. It is named after metallurgist Adolf Martens (1850-1914), who discovered its structure under his microscope during his metallographic research and explained how the physical properties of different types of steel were affected by their microscopic crystalline structures. Martensite commonly is found in tools such as hammers and chisels and in swords.
How is martensite formed?
Equilibrium phases form by slow cooling rates allowing sufficient time for diffusion, whereas martensite is usually formed by fast cooling rates. Since chemical processes (the attainment of equilibrium) accelerate at higher temperature, martensite is easily destroyed by the application of heat.
What is the difference between austenite and martensite?
One of the differences between the two phases is that martensite has a body-centered tetragonal (BCT) crystal structure, whereas austenite has a face-centered cubic (FCC) structure. Martensite has a lower density than austenite, so that the martensitic transformation results in a relative change of volume.
Do martensite plates have crystal orientations?
A crystallographic analysis has shown that the martensite plates have very definite crystal orientations with respect to the original structure. These orientation relationships can nowadays well be accounted for by phenomenological theories, described first by Wechsler, Lieberman and Read, and Bowles and Mackenzie, discussed in the book by Nishiyama and in the book edited by Otsuka and Wayman.
Why is martensite not shown in the equilibrium phase diagram of the iron-carbon system?
Martensite is not shown in the equilibrium phase diagram of the iron-carbon system because it is not an equilibrium phase. Equilibrium phases form by slow cooling rates allowing sufficient time for diffusion, whereas martensite is usually formed by fast cooling rates.
Abstract
A novel concept for the heat treatment of martensite, different to customary quenching and tempering, is described. This involves quenching to below the martensite-start temperature and directly ageing, either at, or above, the initial quench temperature.
1. Introduction
Conventional quenching and tempering heat treatments have long been applied to steels to produce good combinations of strength and toughness from the martensitic structure (e.g. [1] ).
2. Background and quench and partitioning fundamentals
Although the existence of carbon-enriched retained austenite in martensitic steel microstructures has been known for some time [23], [24], [25], the process of partitioning from the supersaturated martensite to the untransformed austenite has received little attention because the essential elimination of carbon supersaturation in the martensite is ordinarily accomplished by carbide precipitation during tempering [26].
3. Experimental observations and applications
A number of experimental and commercial steel compositions that have so far been examined are given in Table 1. These have been mainly representative of medium carbon bar steels and lower carbon sheet steels, with enhanced Si or Al addition.
4. Summary
The potential to produce useful ferrite/retained austenite microstructures by means of a novel heat treatment route, termed quenching and partitioning, which utilizes the martensite transformation, has been described.
Acknowledgements
This research programme is currently being conducted under an international collaboration supported in the authors’ respective countries by NSF Grant #0303510 (USA), EPSRC Grant ref. GR/S86501 (UK) and CNPq Grant Institutional Process #69.0053/03-7 (Brazil).
What happens to maraging steels after austenitizing?
After the maraging steels are transformed to martensite after austenitizing, they have relatively low strengths. High strengths are developed in these materials only after the martensitic structure is aged. Age hardening of the maraging steels is due to the precipitation of fine distributions of second phase particles. Precipitation strengthening of maraging steels can be accomplished in a number of different ways. Transmission electron microscopy of aged steels suggests that the precipitates responsible for the strengthening of the conventional maraging steels (18NiCo (200), 18NiCo (250) and 18NiCo (300)) are Ni3 Mo ( Decker and Floreen 1988 ). While cobalt is not contained in the strengthening precipitates, cobalt additions play an important role in achieving high strength in the cobalt-containing maraging steels. In an extensive study of Fe–Ni–X ternary alloys, Floreen (1964) found that age hardening could be achieved when X was Al, Be, Nb, Mn, Mo, Si, Ta, Ti, or W. When the effects of these elements are compared at the same atomic percent, titanium was found to have the greatest effect on strength. Notably, Fe–Ni–Co alloys did not exhibit age hardening at cobalt levels less than about 4.5 at.%, and only modest age hardening at levels as high as 15 at.%. Floreen and Speich (1964) compared the age hardening behavior of Fe–Ni–Co–X quaternary alloys (where X was Al, Be, Nb, Mn, Mo, Si, and Ti) to the results obtained by Floreen for the ternary alloys and found that the cobalt considerably enhanced the age hardening due to molybdenum, but did not have a similar effect on the age hardening by the other elements considered. Floreen and Speich (1964) observed that cobalt additions led to a much finer dispersion of particles of Ni 3 Mo and suggested that this could be due to cobalt reducing the solubility of Ni 3 Mo in the martensite. In his review article, “Physical Metallurgy of Maraging Steels,” Floreen (1968) provides references to precipitate identification for a number of different maraging systems. Peters and Cupp (1966) have carried out extensive studies of the kinetics of aging reactions of Fe–Ni, Fe–Ni–Mo, Fe–Ni–Co–Mo, and related maraging compositions.
What is hardening steel?
Hardening of steel means, in general, formation of a martensitic structure by heat treatment in solid state. This structure has higher strength but less ductility and is more resistant against many kinds of wear. At room temperature pure iron consists of ferrite with a body-centred cubic (bcc) structure, also called α-iron. Having 0.9% carbon steel consists of perlite. This is a eutectoid mixture of ferrite and zementite (Fe3 C). Perlite forms grains which consist of lamellar ferrite and zementite. With a carbon content below 0.9% steel is a mixture of ferrite and perlite. At higher carbon content between 0.9% and 1.7% secondary zementite and perlite are structural components of steel. At room temperature ferrite can dissolve only a little carbon. Between the temperatures Ac 1 and Ac 3 the transformation of ferrite into austenite with a face-centred cubic (fcc) structure occurs. Ac 3 is 906 °C for pure iron and 721 °C for steel with 0.9% carbon. Austenite can dissolve up to 1.7% carbon. Holding steel at temperatures above Ac 3 leads to diffusion of carbon into austenite. At slow cooling the carbon diffuses back and austenite transforms to perlite. But quenching the steel keeps the carbon in the austenite structure and the lattice transformation from face-centred cubic to body-centred cubic cannot occur completely. The result is the so-called martensitic structure and some remaining austenite. Martensite is very hard and needle-like in cross-section. At less abrupt quenching a certain amount of perlite is nevertheless formed. Alloying elements have a considerable influence on the above-mentioned transformations. They form carbides, for instance, which are less soluble and reduce the critical quenching rate. Higher alloyed steels need more time and temperature for complete austenite formation, carbon solution, and diffusion, in general. But, on the other hand, alloying elements are often an agent that martensite forms even at lower quenching rates. It is important to know that for some steels the martensitic transformation is not completed at room temperature. Sometimes liquid nitrogen cooling is applied. More details on structural changes and transformation behaviour is given in [ 1 ]. The characteristic behaviour of the different types of steel during cooling from austenite is described in time-temperature-transformation-charts. But these charts are developed for classical heat treatment. For laser short-time processes these charts are a good estimation but have to be applied creatively. The main difference is the much higher surface temperature compared to classical heat treatment. The transformation and diffusion processes must be accelerated by higher temperatures because the interaction time of the laser at a given position is very short [ 2, 3 ]. Typical surface temperatures at laser beam hardening are, depending on the material, in the range of 1100–1400 °C.
How is steel tempered?
The steel is normally tempered after thermal hardening by heating between 150 and 650 °C (300 and 1200 °F) dependent upon the material properties and the specific mechanical properties required. Tempering can continue up to the lower critical temperature of 723 °C, at which point most of the extra hardness produced by thermal hardening will have been removed, but the fine grain structure produced by the hardening process will remain. Quenched and tempered (QT) steels are normally tempered from between 550 and 650 °C giving them good toughness and strength
What is the carbon content of perlite?
Perlite forms grains which consist of lamellar ferrite and zementite. With a carbon content below 0.9% steel is a mixture of ferrite and perlite. At higher carbon content between 0.9% and 1.7% secondary zementite and perlite are structural components of steel. At room temperature ferrite can dissolve only a little carbon.
What is HICC in steel?
HICC may occur in the HAZ of all hardenable steels (i.e. C, C/Mn) or in the weld metal of high strength low alloy (HSLA) steels that are microalloyed with small amounts of titanium, vanadium or niobium (typically < 0.05%). The hydrogen breaks down at increased temperatures into atomic hydrogen (which has a small atomic size) and escapes to the atmosphere through the steel microstructure. When the temperature reduces to below around 300 °C the hydrogen starts reforming to the hydrogen element and will no longer be able to escape from the material. As the H 2 reforms it may build up an internal pressure stress within the material structure itself.
What is steel made of?
With a carbon content below 0.9% steel is a mixture of ferrite and perlite. At higher carbon content between 0.9% and 1.7% secondary zementite and perlite are structural components of steel. At room temperature ferrite can dissolve only a little carbon.
What is heat treatment?
Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating ...
What is a heat treating furnace?
Heat treating furnace at 1,800 °F (980 °C) Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass.
What happens when an alloy is cooled to an insoluble state?
If the alloy is cooled to an insoluble state, the atoms of the dissolved constituents (solutes) may migrate out of the solution. This type of diffusion, called precipitation, leads to nucleation, where the migrating atoms group together at the grain-boundaries.
What is the temperature of steel?
For instance, steel that has been heated above the austenizing temperature (red to orange-hot, or around 1,500 °F (820 °C) to 1,600 °F (870 °C) depending on carbon content), and then cooled slowly, forms a laminated structure composed of alternating layers of ferrite and cementite, becoming soft pearlite.
What is the melting point of a hypoeutectic alloy?
A hypoeutectic alloy has two separate melting points. Both are above the eutectic melting point for the system but are below the melting points of any constituent forming the system. Between these two melting points, the alloy will exist as part solid and part liquid. The constituent with the lower melting point will solidify first. When completely solidified, a hypoeutectic alloy will often be in a solid solution.
What is annealing in metals?
Annealing consists of heating a metal to a specific temperature and then cooling at a rate that will produce a refined microstructure, either fully or partially separating the constituents. The rate of cooling is generally slow. Annealing is most often used to soften a metal for cold working, to improve machinability, or to enhance properties like electrical conductivity .
Why are metals annealed?
Most non-ferrous alloys that are heat-treatable are also annealed to relieve the hardness of cold working.
How is martensite formed?
Martensite is formed in carbon steels by the rapid cooling ( quenching) of the austenite form of iron at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe 3 C). Austenite is gamma-phase iron (γ-Fe), a solid solution of iron and alloying elements.
What is the name of the hard form of steel?
Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation.
What is heat treatment?
Heat treatment involves heating of metal in the solid-state and then subsequently cooled at varied cooling rates. It is very important manufacturing process that can not only help the manufacturing process but can also improve the product, its performance, and its characteristics in many ways. By Heat Treatment process, Example: The plain carbon ...
What temperature does annealing take place?
Annealing consists of heating of steel parts to a temperature at or near the critical temperature 900 degree Celsius hold it at that temperature for a suitable time and when allowed to cool slowly in the Furnace itself. The heating done during annealing affects the metal in two stages of recovery and recrystallization.
What is annealing in metal?
Annealing is carried out for accomplishing one or more of the following: Softening of a metal or alloy. This may be done due to improving machinability. Relieving internal residual stresses caused by the various manufacturing process. Refining the grain size of the metal or alloy.
What is recrystallization in steel?
This causes complete recrystallization in steel to form New grain structure. This will release the internal stresses previously the strip in the steel and improve the machinability.
What is normalizing steel?
Normalizing is a heat treatment process similar to annealing in which the Steel is heated to about 50 degree Celsius above the upper critical temperature followed by air cooling. This results in a softer state which will be lesser soft than that produced by annealing.
What is the purpose of hardening steel?
Hardening is carried to accomplish the following: To reduce the grain size. Obtain maximum hardness.
What is nitriding used for?
Nitriding is generally employed to Steel parts which are moving like engine parts such a cylinder, crankshaft, etc. 6. Cyaniding: Cyaniding is also a surface hardening process in which the heated parts to be surface hardened are immersed in a bath of molten sodium or potassium cyanide.