
What does heat treatment do to metals?
A heat treatment can effectively make a metal have a certain level of electrical resistance. The reason that this happens is that when metals are heated, their electrons can absorb addition energy and makes them move faster than normal. 4. Reduces a Metal’s Magnetism
What happens to metals when they are heated?
Metals heated to certain temperatures also can lose their magnetism. By raising temperatures to between 626 degrees Fahrenheit and 2,012 degrees Fahrenheit, depending on the metal, magnetism will disappear. The temperature at which this happens in a specific metal is known as its Curie temperature.
Do heat treated metals become harder or softer over time?
Depending on the method used, heat treated metals become harder or softer, more or less brittle, or stronger or weaker. Based on the desired end results, the method may involve:
What happens if you don’t pack heat treated metal properly?
Parts such as rods can become bowed if they expand and are not properly packaged after metal heat treating. Other parts can start rubbing together and get scratches in their surface finish if the packaging no longer holds the heat treated metal parts securely.
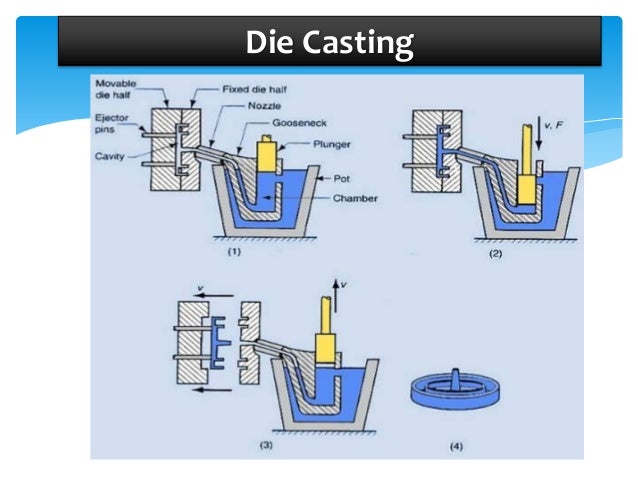
What happens during heat treatment?
Heat treatment is the process of heating metal without letting it reach its molten, or melting, stage, and then cooling the metal in a controlled way to select desired mechanical properties. Heat treatment is used to either make metal stronger or more malleable, more resistant to abrasion or more ductile.
How heat treatment can change the properties of metals?
How Does Heat Treatment Affect the Properties of Metals?Thermal Expansion. As metals are heated, their volume, surface and length will expand. ... Structural Alterations. ... Makes the Metals Resistant to Electrical Current. ... Reduces a Metal's Magnetism.
What happens to steel after heat treatment?
Heat Treatment Steel: Hardening While hardening does increase strength, it also decreases ductility, making the metal more brittle. After hardening, you may need to temper the metal to remove some of the brittleness.
Why do metals expand when heated?
What does heat do to metal? The expansion (or contraction) of any material is due to the kinetic energy of its atoms. When a material is heated, the increase in energy causes the atoms and molecules to move more and to take up more space— that is, to expand. This is true of even a solid such as a metal.
What properties does heat treatment change?
Heat treating is often used to alter the mechanical properties of a metallic alloy, manipulating properties such as the hardness, strength, toughness, ductility, and elasticity.
Does metal shrink when heat treated?
In another example, according to a Latrobe Steel data sheet, 17-4 precipitation hardening stainless steel can typically be expected to shrink by 0.0004 to 0.0006 inch/inch (size change per unit of length) when aging from Condition A to Condition H-900 and 0.0018 to 0.0022 inch/inch when aging from Condition A to ...
What happens to the steel part when quenched?
Quench Hardening Steel Through a quenching process known as quench hardening, steel is raised to a temperature above its recrystallization temperature and rapidly cooled via the quenching process. The rapid quenching changes the crystal structure of the steel, compared with a slow cooling.
What happens to metals during annealing?
Annealing makes metals more formable. When metal is stronger and more ductile, it gives manufacturers more leeway in the fabrication process. There is less risk of material fracturing from bending or pressing. Annealing can also improve a metal's ability to be machined and improve the lifespans of tools.
What happens to metals when they are heated?
The actual structure of metal also changes with heat. Referred to as allotropic phase transformation, heat typically makes metals softer, weaker, and more ductile. Ductility is the ability to stretch metal into a wire or something similar. Heat also can impact the electrical resistance of metal.
Why do metals need heat treatment?
The most common reasons that metals undergo heat treatment are to improve their strength, hardness, toughness, ductility, and corrosion resistance. Common techniques for heat treatment include the following: Annealing is a form of heat treatment that brings a metal closer to its equilibrium state.
What temperature does a metal lose its magnetism?
Metals heated to certain temperatures also can lose their magnetism. By raising temperatures to between 626 degrees Fahrenheit and 2,012 degrees Fahrenheit, depending on the metal, magnetism will disappear. The temperature at which this happens in a specific metal is known as its Curie temperature.
What is heat treatment?
Heat treatment is the process of heating and cooling metals to change their microstructure and to bring out the physical and mechanical characteristics that make metals more desirable. The temperatures metals are heated to, and the rate of cooling after heat treatment can significantly change metal's properties.
How long does it take for precipitation hardening to take place?
It can take anywhere from an hour to four hours to carry out the process. The length of time typically depends on the thickness of the metal and similar factors.
Why is tempering used in steelmaking?
Commonly used in steelmaking today, tempering is a heat treatment used to improve hardness and toughness in steel as well as to reduce brittleness. The process creates a more ductile and stable structure.
What is the process of quenching metal?
The quenching process stops the cooling process from altering the metal's microstructure.
Why is heat treatment important in metal manufacturing?
This is because it helps to improve a metal part to withstand wear and tear better.
Why use heat treated metal?
Using effectively heat-treated metal parts ensures the effective and cost-effective running of machines. Furthermore, the product will be a lot more efficient, even for the toughest applications. Also, there may be the need for extremely hard metals for some applications.
Why do we heat treat steel?
This is another heat treatment process that helps to increase the resilience of steel. Iron-based alloys are usually hard but often too brittle for certain applications. Tempering helps to alter the hardness, brittleness, and ductility of the metal. This is in a bid to make the machining process easier.
What are the benefits of heat treatment?
In a nutshell, the benefits of heat treatment of metals include: Increases strength, making the material ductile or more flexible. It introduces wear-resistant properties to the metal. Relieves stresses, making the part easier to machine or weld.
What is hardening metal?
Hardening. Hardening involves the heating of the metal material to a specific temperature. This temperature is the point at which the elements present in the metal goes into solution. The crystal lattice structure of the metal may have defects that present a source for plasticity.
What is the process of making metal harder?
This usually made the metal a lot harder and less brittle. This is a basic process called heat treatment of metals. Modern machining and metalworking processes are now more precise and sophisticated. Many different techniques help shape metals for various purposes.
Why does cooling occur?
Then, cooling occurs to harden the heated material. The process aims towards changing the microstructure of the metal. Also, it helps to bring out desired mechanical, chemical, and physical characteristics. The alteration of these properties benefits the working life of the component involved.
What are the effects of heating metals?
The Effects of Heating Metals. 1. Thermal Expansion. As metals are heated, their volume, surface and length will expand. The term for these actions is thermal expansion. Each metal will have a different rate of expansion when exposed to the heat. 2. Structural Alterations. Another effect that heat treatments have on metals is that the structure ...
How does heat affect metals?
This is due to the fact that heat displaces the allotrope atoms in metals and causes them to reform in a different configuration . For this reason, this action is called the allotropic phase transformation.
Why does heat treatment make metals resist?
The reason that this happens is that when metals are heated, their electrons can absorb addition energy and makes them move faster than normal. 4.
Why do we use heat treatment?
The most popular reasons for performing these treatments is to increase a metal’s toughness, hardness, strength, corrosion or electrical resistance, and ductility.
How does annealing soften metal?
The following are the most common methods for performing these treatments: • Annealing softens the metal through heating to make it workable and to increase its ductility. The metal is heated to the appropriate temperature to alter its microstructure and then, it is slow-cooled.
Is steel brittle?
Steel that does not undergo this process is extremely hard but too brittle to use in many applications. While there are many other details to learn about how heat treatments affect the properties of metals, the above information gives you a start on your education about this topic.
How to heat treat metal?
Depending on the method used, heat treated metals become harder or softer, more or less brittle, or stronger or weaker. Based on the desired end results, the method may involve: 1 Using several treatments 2 Altering the temperature at which the metal is heat treated 3 Varying the length of time heat is applied 4 Controlling how quickly or slowly the material is cooled
Why is it important to make sure heat treated metal parts are packaged properly?
In addition, it is important to make sure heat treated metal parts will be packaged properly to avoid distorting or damaging the previously cut parts while they are in transit.
What is annealing metals?
Often used interchangeably with the term heat treating , annealing is a specific method used to soften metals, with the goals of increasing their ductility and decreasing brittleness . Annealing can also be used to increase the homogeneous nature of metals, as well as to restore their ductility prior to further handling.
Why is annealing used?
This technique is used to improve the hardness and durability of products such as carbon steel wire springs and forgings. However, if “carburization” is not a desired trait, annealing should be performed in an environment that is low in or free of carbon.
What happens when titanium atoms combine with oxygen?
For instance, if the titanium atoms combine with oxygen or carbon, the NiTi crystal structure can lose titanium, causing the transformation temperature to be lowered. If there is too little nickel and the material is aged for too long, the transformation temperature is increased.
Why do metal rods bowed?
Parts such as rods can become bowed if they expand and are not properly packaged after metal heat treating. Other parts can start rubbing together and get scratches in their surface finish if the packaging no longer holds the heat treated metal parts securely.
Why are annealed parts warped?
Since parts are made more pliable by annealing, inadequate packaging could cause annealed parts to become warped when they are repackaged and sent to you (or sent back to us for additional processing). Parts such as rods can become bowed if they expand and are not properly packaged after metal heat treating.
How does heating a metal affect its hardness?
This process is known as allotropic phase transformation. Allotropic phase transformation alters the hardness, strength and ductility of the metal. The most important allotropic phase transformation is undergone by iron. When iron is heated past 1,674 degrees Fahrenheit it is able to absorb more carbon, which is an ingredient that will increase the hardness of any steel product. This desired effect is used in several types of High Carbon (above 0.50 carbon) steel – Example: Tool Steel
Why does metal expand when heated?
Length, surface area and volume will increase with temperature. The scientific term for this is thermal expansion. The degree of thermal expansion varies with different types of metal. Thermal expansion occurs because heat increases the vibrations of the atoms in the metal.
How does annealing affect metal?
Annealing alters the physical and chemical properties of the metal to increase ductility and reduce hardness. This facilitates shaping, stamping or forming processes, and allows the metal to be cut more easily. Annealing also enhances electrical conductivity.
How does annealing work?
Annealing is frequently used to soften metals including iron, steel, copper, brass and silver. The process involves heating the metal to a specific temperature then allowing it to cool slowly at a controlled rate. Annealing alters the physical and chemical properties of the metal to increase ductility and reduce hardness. This facilitates shaping, stamping or forming processes, and allows the metal to be cut more easily. Annealing also enhances electrical conductivity.
What is tempered steel?
Untempered steel is very hard but too brittle for most practical applications. Tempering is a low temperature heat treatment process normally performed after hardening (neutral hardening, double hardening, atmospheric carburising, carbonitriding, or induction hardening) in order to reach a desired hardness/toughness ratio. The process involves heating steel to a lower temperature to reduce some of the excess hardness. The metal is then allowed to cool in still air which results in a tougher and less brittle steel.
What is hardening steel?
Hardening. Hardening is applied to steel and other alloys to improve their mechanical properties. During hardening, the metal is heated at a high temperature and this temperature is maintained until a proportion of carbon has been dissolved. Next the metal is quenched, which involves rapidly cooling it in oil or water.
What are the three metals that have magnetic properties?
There are three metals with magnetic properties: iron, nickel and cobalt. They are known as ferromagnetic metals. Heating these metals will reduce their magnetization to the point where magnetism is completely eradicated. The temperature at which this occurs is known as the Curie temperature.
Why is metal treated in advance?
Treated metal that requires too much heat affects the toughness and strength of the metal.
What happens if you don't temper metal?
If you don't temper your metal properly, you'll expose your metal products to potential brittleness. Brittleness occurs when metal is tempered for too short of a time. Lower your hardening temperature and allow for proper tempering and you'll avoid brittleness.
How to avoid cooling fractures?
If you want to avoid cooling fractures, first consider the hardness capacity of the metal you're dealing with. Select your tools based on the amount of hardening and shape that you're dealing with. Fractures occur during the quenching process often.
How to prevent warping metals?
In order to avoid warping your metals before they're treated, the metal treatment has to be normalized. Maintain even temperatures throughout and provide the right amount of treatment time in advance. Your furnace temperature must be maintained throughout the entirety of your treatment process. Metal must be stabilized to prevent movement .
What temperature does decarburization take?
Decarburization is often the culprit for negatively impacting or destroying your steel. When your temperature goes above 1200° C, your steel or low allow metals are up for being damaged. The mechanical properties of your metals become subject to deterioration when heat treatment comes before the forging process.
Why is it important to stabilize metal before quenching?
Metal must be stabilized to prevent movement. This is especially important during the quenching process if you want to avoid warping. Time each step as you go along. Proper timing ensures that each step is completed properly. Stressing metal before the treatment process introduces issues as well.
What are the factors that affect the impact strength of metals?
A variety of factors affect the impact strength of metals that have been treated. The number and type of impurities in a metal are one of the most important factors in determining the success of heat treatment.
What temperature does steel ferrite melt?
Using between 750°C and 980°C (the temperature varies because of carbon content) of furnace heat, all of the steel ferrite is transformed into a harder, uniformly distributed pearlite structure.
How does steel change its grain structure?
In steel workpieces, the grain structure of the component changes size. Alternatively, new grains form. They undergo phase transformative processes. There’s carbon in the mix, too. As those phase changes take place, the alloy-strengthening element becomes more soluble. The carbon diffuses, so the workpiece hardens.
Can ferritic steel diffuse carbon?
Body-centred ferritic steels can’t easily diffuse carbon. Infusing more thermal energy into the process, taking the temperature gradient up to that 750 to 980°C sweet spot, the grain structure transforms. Face-centred austenite allows the carbon to diffuse. Hard islands of cementite dissolve, and now the crucial moment has arrived. If the second half of the heat treatment process mirrors the first half, then the carbon will resurface while the steel assumes its ferritic microcrystalline structure once more. Obviously, that’s not the result we’re after. Taking control of that second stage, the cherry-hot steel is quenched in oil or water. Now, because of the sudden cooling, the carbon becomes locked inside the cubic grains.
