Types of chlorine used in water treatment:
- Chlorine gas: You may have heard of chlorine gas being used as a deadly weapon during WWII. ...
- Calcium hypochlorite: Chlorine hypochlorite is the most common compound of chlorine used in residential water treatment. ...
- Sodium hypochlorite: Easiest to handle of all three compounds, sodium hypochlorite can most easily be recognised as household bleach.
What is the acceptable level of chlorine in water?
To perform the chlorine test:
- Remove the test strip from the packet.
- Pick up the strip at the end with no pads.
- Dip the strip in the sample three times and remove without shaking off excess water.
- Hold the test strip level for two seconds.
- Place the strip on the results color chart to determine the precise amount of chlorine in your water sample.
Is it safe to drink water with chlorine?
Is It Safe to Drink Water with Chlorine? If regulations are followed, then yes. The U.S. Environmental Protection Agency (EPA) limits the amount of chlorine in drinking water to levels considered safe for human consumption and are unlikely to cause adverse long-term health effects.
How much chlorine is safe to drink?
The Environmental Protection Agency (EPA) along with the CDC has determined that the safe level of chlorinated drinking water is 4 milligrams per liter which can also be termed 4 parts per million (ppm).
What are the side effects of chlorine?
Symptoms of this problem can include:
- changes in the pH balance of your blood
- low blood pressure
- serious injury to the eyes, including blurry vision, burning, irritation, and in extreme cases vision loss
- skin damage, resulting from tissue injury with burns and irritation
See more
Why is chlorine used as a disinfectant?
Which is more effective for disinfecting: chlorine or hypochlorite?
What is the best way to disinfect water?
What is calcium hypochlorite?
How much chlorine is needed for disinfection?
What is the best way to provide safe water to the end user?
Which is the least expensive chlorine?
See more
What chlorine is used in water treatment?
The three most common chlorine-containing substances used in water treatment are chlorine gas, sodium hypochlorite, and calcium hypochlorite. The choice of the chlorine type to be used often depends on cost, on the available storage options and on the pH conditions required.
What form is chlorine used in?
There are 5 types of Chlorine; Sodium hypochlorite, Lithium hypochlorite, Calcium hypochlorite, Dichlor, and Trichlor. The first difference is Sodium, Lithium, and Calcium are un-stabilized Chlorine. Dichlor and Trichlor are stabilized.
Why is chlorine used in water treatment?
Besides killing dangerous germs like bacteria, viruses and parasites, chlorine helps reduce disagreeable tastes and odors in water. Chlorine also helps eliminate slime bacteria, molds and algae that commonly grow in water supply reservoirs, on the walls of water mains and in storage tanks.
Which is better dichlor or Trichlor?
Trichlor slowly dissolves and is ideal for daily chlorination, is cheaper and is easier to maintain. Trichlor is more of a set and forget method with its tablets. What is this? Dichlor is better for raising your levels of chlorine and cyanuric acid quickly.
What is chlorine used for?
Chlorine is most commonly used in water treatment for as a disinfectant, though seldom in its pure form. Chlorine gas, sodium hypochlorite and calcium hypochlorite are the three most common chlorine compounds used in water treatment.
When was chlorine first used in water?
The use of chlorine in water treatment has been around since 1893 and permanent water chlorination began in 1905. Hamburg, Germany was the first to attempt chlorinating drinking water, shortly followed by Maidstone, England, as a way to make water completely germ-free.
What is the role of calcium hypochlorite in food?
Calcium hypochlorite also plays a major role in industrial food processing, killing germs and keeping our food supplies safe. For example, if you have water stored in a large tank, adding the right about of calcium hypochlorite with the help of a chlorination system will help disinfect the water.
What is the most common compound used in water treatment?
Calcium hypochlorite: Chlorine hypochlorite is the most common compound of chlorine used in residential water treatment. Calcium hypochlorite increases the pH of the water it is being used to treat and can be sold as calcium hypochlorite pellets or granules. Calcium hypochlorite should also be handled with care as it is capable ...
What happens during the disinfection stage of water treatment?
During the disinfection stage of the water treatment process – most of the time, chlorination occurs at this stage. Coagulation: untreated water enters the treatment plant and liquid aluminium sulphate is added, causing tiny dirt particles in the water to stick together.
What is chlorination in water?
Chlorination is a water treatment process that is used to eliminate certain pathogens, particularly in drinking water, such as dysentery and typhoid. Water treatment processes such as coagulation, filtration and sedimentation are used to battle waterborne diseases. For over a century, however, chlorination has been used to create water ...
When is chlorination performed?
Chlorination can also be performed during any part of the water treatment process, including: Pre-chlorination – almost immediately after water enters the treatment facility. After sedimentation and prior to filtration. During the disinfection stage of the water treatment process – most of the time, chlorination occurs at this stage.
When was chlorine first used?
Chlorine was first used in the United States as a major disinfectant in 1908 in Jersey City, New Jersey. Chlorine use became more and more common in the following decades, and by 1995 about 64% of all community water systems in the United States used chlorine to disinfect their water.
What is the process of adding chloramine to drinking water to disinfect it and kill germs?
Chloramination is the process of adding chloramine to drinking water to disinfect it and kill germs. It is sometimes used as an alternative to chlorination. Chloramines are a group of chemical compounds that contain chlorine and ammonia.
What is the best disinfectant for drinking water?
Several major U.S. cities such as Philadelphia, San Francisco, Tampa Bay, and Washington, D.C. use chloramine to disinfect drinking water. Chloramine is recognized as a safe disinfectant and a good alternative to chlorine.
What is the EPA's water treatment system?
The U.S. Environmental Protection Agency (EPA) allows drinking water treatment plants to use chloramine and chlorine to disinfect drinking water. Water system pipes develop a layer of biofilm (slime) that makes killing germs more difficult.
What is the EPA's hotline for chloramine?
EPA provides guidance for local water authorities switching to chloramine on how to minimize lead and copper levels. If you are concerned about lead or copper levels in your household water, call EPA’s Safe Drinking Water Hotline at 800-426-4791 for testing information.
Where is chloramine used?
Chloramine has been used as a drinking water disinfectant in the United States in places like Cleveland, Ohio, Springfield, Illinois, and Lansing, Michigan since 1929. In 1998, an EPA survey estimated 68 million Americans were drinking water disinfected with chloramine.
What is the purpose of water in dialysis?
During dialysis, large amounts of water are used to clean waste products out of a patient’s blood. Dialysis centers must treat the water to remove all chemical disinfectants, including chlorine and chloramine, before the water can be used for dialysis.
What is chlorine used for in disinfection?
Chlorine reacts with organic matter to disinfection byporducts, such as trihalomethanes (THM) and halogenated acetic acids (HAA). Chlorine can be added for disinfection in several different ways. When ordinary chlorination is apllied, the chlorine is simply added to the water and no prior treatment is necessary.
When was chlorine first made?
Chlorine (Cl2) was first prepared in pure form by the Swedish chemist Carl Wilhelm Scheele in 1774. Scheele heated brown stone (manganesedioxide; MnO2) with hydrochloric acid (HCl). When these substances are heated the bonds are broken, causing manganese chloride (MnCl2), water(H2O) and chlorine gas (Cl2) to form.
What is the pH of water for disinfection?
The effectivity of disinfection is determined by the pH of the water. disinfection with chlorine will take place optimally when the pH is between 5,5 and 7,5. underchloric acid (HOCl) reacts faster than hypochlorite ions (OCl-); it is 80-100% more effective. The level of underchloric acid will decrease when the pH value is higher.
How is chlorine produced?
Finally, chlorine can be produced by means of molten salts electrolysis and, mainly in laboratories, by means of hydrochloric acid and manganesedioxide oxidation: MnO2+ 4HCl -> MnCl2+ 2H2O + Cl2. When gaseous chlorine is added to water the following hydrolysis reaction takes place: Cl2+ H2O = H++ Cl-+ HOCl.
How is chlorine broken down?
Chlorine is broken down under the influence of sunlight. UVradiation in sunlight provides energy which aids the break-down of underchloric acid (HOCl) molecules. First, the water molecule(H2O) is broken down, causing electrons to be released which reduce the chlorine atom of underchloric acid to chloride (Cl-).
What disinfectant is used for water disinfection?
Concentrations- Effectivity- Health Effects- Legislation. Chlorine. Chlorineis one of the most commonly used disinfectants for water disinfection. Chlorine can be applied for the deactivation of most microorganisms and it is relatively cheap.
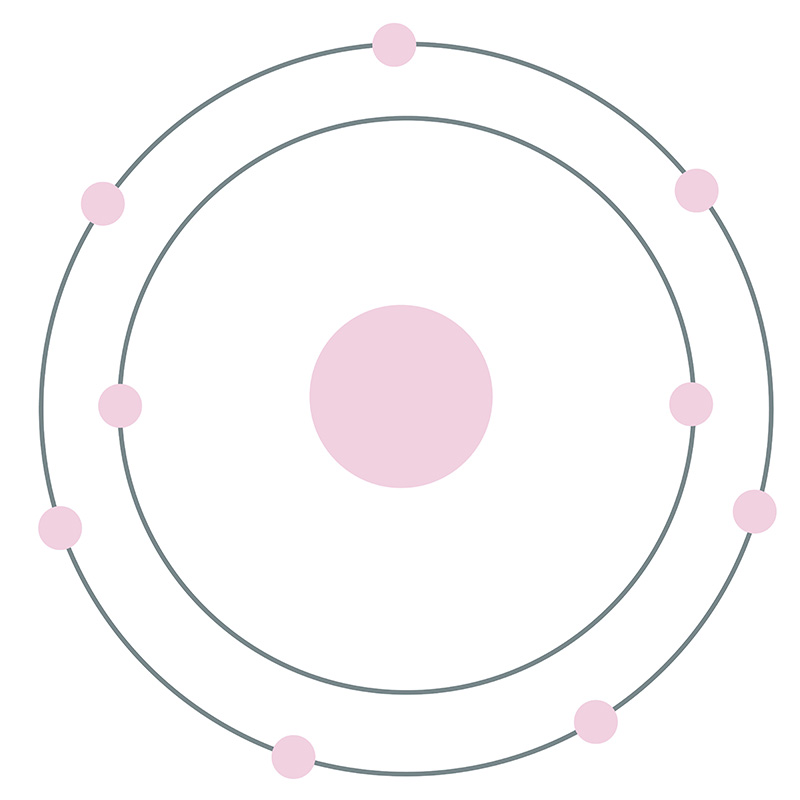
How many electrons does chlorine have?
It can also cause an extra eletron to form (a covalent bond; a chlorine bond), causing the outer shell to complete. Figure 2: chlorine atoms contain 17 electrons. Chlorine can form very stable substances, such as kitchen salt (NaCl). Chlorine can also form very reactive products, such as hydrogenchloride (HCl).
Why is chlorine used in disinfecting water?
for years, mainly because of its cost-effectiveness, ease of use, wide-scale availability, and proficiency at destroying most pathogens that cause some of the most dangerous waterborne illnesses today.
How to treat chlorinated water?
Our recommended approach to treating chlorinated water is filtration. By running the water through a filter with activated charcoal in granular or particle form, you can significantly reduce the chlorine and chloramine contents in your water, as well as the general taste and odor associated with chlorine and DBPs.
Why is chlorine bad for you?
The high toxicity of chlorine makes it a powerful chemical that can destroy bacteria, microbes, and pathogens that can leach into your water supply. By killing these disease-causing germs, the compound helps to make water safe to drink. Waterborne diseases have killed thousands of U.S. residents every year.
Why is chlorine added to water?
When chlorine is added to your water supply, it rapidly reduces the spread of all kinds of waterborne diseases, like cholera and typhoid fever, as well as other ailments. It also makes it easier for cities and towns to purify drinking water to keep residents (like yourself) safe.
What is the name of the chemical that is added to pool water?
Instead, liquid chlorine or sodium hypochlorite (NaOCl) is added to the pool water. When either of these forms of chlorine is pumped into the water, it creates hypochlorous acid (HOCl), which is highly active against all bacterial, viral, and fungal human pathogens.
How to find out how much chlorine is in water?
The fastest way to find out is to either request a water quality report from your local municipality or purchase a DIY home water test kit and check your water for chlorine.
What is the most common disinfectant used in water?
Chemical purification. Chlorine is by far the most commonly used water disinfectant worldwide. Today, about 98% of U.S. municipalities use some chlorine- related process to treat their drinking water, thanks to the chemical’s wide-scale availability, low cost, ease of use, and proficiency at destroying germs.
Why are the chemicals used for water treatment important?
Nowadays, the demand for potable water is constantly increasing, due to meeting human needs and supporting industrial activities. With increasing urbanization and economic development, the current water supply is unlikely to meet the ever-increasing demands.
The chemicals used in water treatment are
In addition to the chemicals mentioned above, there are many other chemicals used in water treatment. Coagulants, flocculants, softeners, and filter cleaners also form an important part of water treatment methods.
Aluminum sulfate
Aluminum sulfate is the main substance that helps condense pollutants in water. And adding slaked lime to adjust the pH of the water to get the best effect after thickening. Polyelectrolytes are used for condensation. It uses chlorine in addition to activated carbon.
The importance of water treatment
The water treatment industry plays an important role in providing clean water and preventing various water-related diseases across the world. With the help of innovative boiler water treatment chemicals and other related chemicals. It is now possible to make polluted seawater, river waste, and sewage safe for human consumption.
Coagulation and flocculation
The two are usually the first steps in water treatment in which positively charged chemicals are added to the water. The positive charge of these chemicals neutralizes the negative charge of dirt and other dissolved particles in the water. When this happens, the particles combine with the chemicals and form larger particles called agglomerates.
Sedimentation
The conglomerates settle due to their weight at the bottom of the water source during sedimentation. This process is called sedimentation, where the sedimentation or sediment resulting from the coagulation and flocculation process takes place in the subsequent stages.
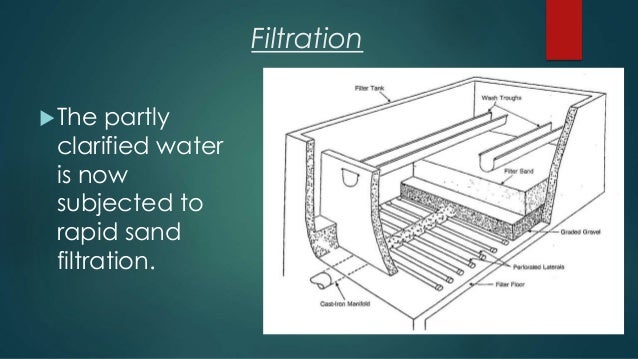
Purification
After removing the sludge, we enter the filtration stage. During this process, the sludge passes through the material layer, which helps to remove organic matter and particles that were not removed before by coagulation. The material used is usually a layer of sand over a layer of gravel.
Why is chlorine used as a disinfectant?
Chlorine and pH. In general terms, the lower the pH of the water, the more effective chlorine is as a disinfectant. Again, speaking generally, a reason for dosing effectively is that chlorination raises the pH of water, so overdosing often raises the pH to levels where chlorine does not work effectively as a disinfectant.
Which is more effective for disinfecting: chlorine or hypochlorite?
Chemically, this has to do with the relationship between the two constituents of chlorine that together are often referred to as “free chlorine”—hypochlorus acid and hypochlorite ions. Hypochlorus acid is the more effective disinfectant and it dominates at lower pH levels, so a lower pH is preferred for disinfection.
What is the best way to disinfect water?
Other methods of disinfection such as ultraviolet and ozonation are effective disinfectants but they do not provide a residual to prevent pathogen regrowth as chlorination does. When treatment plants are distant from the point of use, chlorination is the best way to provide safe water to the end user. Municipal water providers usually rely on measurements of “chlorine residual”—the amount of chlorine remaining in the water after it reaches its destination—as proof of safety. Residual requirements vary, but typical residual goal would be for 0.2 to 1 mg/L.
What is calcium hypochlorite?
Calcium hypochlorite is manufactured from chlorine gas. It is best known as chlorine pellets and granules in residential water treatment. It is a white solid with a very pungent odor and it can create enough heat to explode, so it must not be stored near wood, cloth or petroleum products. Calcium hypochlorite increases the pH of the water being treated.
How much chlorine is needed for disinfection?
Residual requirements vary, but typical residual goal would be for 0.2 to 1 mg/L. In addition to disinfection, chlorine can be effectively used to oxidize iron, manganese and hydrogen sulfide to facilitate their removal, to reduce color in water, and to aid in such treatment processes as sedimentation and filtration.
What is the best way to provide safe water to the end user?
When treatment plants are distant from the point of use, chlorination is the best way to provide safe water to the end user. Municipal water providers usually rely on measurements of “chlorine residual”—the amount of chlorine remaining in the water after it reaches its destination—as proof of safety. Residual requirements vary, but typical residual ...
Which is the least expensive chlorine?
Chlorine gas, which is actually sold as an amber-colored compressed liquid, is the least expensive form of chlorine and is, consequently, the preferred type for municipal water systems.