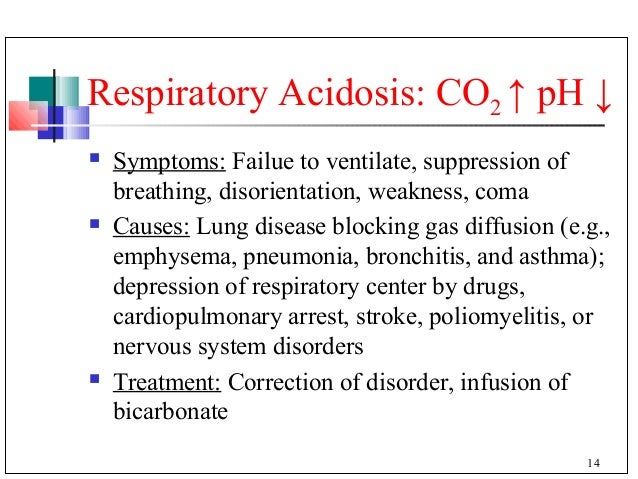
Acetal hydrolysis [H3O+] Definition: Addition of aqueous
Water
Water is a transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's streams, lakes, and oceans, and the fluids of most living organisms. It is vital for all known forms of life, even though it provides no calories or organic nutrients. Its che…
Full Answer
What is addition H3O+?
Acetal hydrolysis [H3O+] Addition of aqueous acid to acetals will transform them back into aldehydes or ketones . This is often referred to as “ deprotection ” of aldehydes or ketones.
Can you drink h_3o+ water?
Addition H 3 O + is a method for adding water (H and OH) across a double bond. This process called hydration. Addition of H3O+ Explained: Alkanes are stable in neutral water so hydration would not occur. But the presence of acid, however, leads to …
What is acetal hydrolysis H3O+?
Sep 03, 2021 · There is a relationship between the H3O+ and OH- concentrations that makes it quite easy to solve one if you know the other. Ion-product Constant for Water (Kw) The formula is: Kw = 1 * 10-14 = (H3O+) * (OH-) The formula for the ion-product constant for water can be rearranged to solve for either H3O+ or OH-.
Is there such a thing as uncharged H3O?
Jul 13, 2008 · usually H3O+ (acid) protonates...depends what the reaction is tho... If you have CH3CH2CH2CN and add H3O+ and heat then you will get a carboxylic acid...so it all depends 0 rdhdds1 Full Member 10+ Year Member Joined Aug 9, 2007 Messages 402 Reaction score 1 Jul 13, 2008 #4 atlanta213 said: what is the role of H3O+ in the reaction? Who, What, Where?
What does H3O+ do in a reaction?
it provides acidic medium to the reaction in the presence of water.Apr 18, 2013
What happens when you add H3O?
0:1520:38Hydration of Alkenes Reaction Mechanism - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe alkene will react with h3o plus the double bond is going to attack the hydrogen atom much theMoreThe alkene will react with h3o plus the double bond is going to attack the hydrogen atom much the same way as a reacts with hbr.
What does h30+ do to an alkene?
Ch 6: Alkene + H3O+ When treated with aq. acid, most commonly H2SO4, alkenes form alcohols. Reaction proceeds via protonation to give the more stable carbocation intermediate.
What does H3O+ do to an alcohol?
15.7: Conversion of Alcohols to Ethers - Symmetrical ethers can be prepared by treating the corresponding alcohol with a strong acid. KMnO4 and chromic acid (Na2Cr2O7, H3O+) oxidize secondary alcohols to ketones, and primary alcohols to carboxylic acids.
What does H3O+ do to carboxylic acid?
Carboxylic acids are sufficiently strong that they ionize to a small extent in aqueous solution. The presence of H3O+ gives rise to the sour taste of vinegar.
What reactions do alkynes undergo?
Alkynes undergo addition reactions due to the presence of loosely held pi-electrons. Due to the presence of a triple bond in alkynes, halogens, water etc.
Is H3O+ an acid catalyst?
When an H+ ion is placed into a solution of water, the electronegative oxygen will act as a nucleophile to grab the H+, giving us a molecule of H3O+, or the hydronium ion, an acid catalyst.Aug 27, 2021
Is H3O+ a catalyst?
The acidic hydronium ion (H3O+) is regenerated in the last deprotonation step, so only a small amount of acid is required to initiate the reaction, the acid therefore is a catalyst.
Is H3O+ an acid or base?
When water acts as a base, it becomes H3O+, which is an acid and is called the conjugate acid of water.Feb 28, 2022
How do you convert ketones to alkenes?
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide (often called a Wittig reagent) to give an alkene and triphenylphosphine oxide.Nov 17, 2016
What is the name of h30+?
Hydronium ionHydronium / IUPAC ID
Do acids Protonate?
Protonation and deprotonation (removal of a proton) occur in most acid–base reactions; they are the core of most acid–base reaction theories. A Brønsted–Lowry acid is defined as a chemical substance that protonates another substance.
What is the name of the cation H3O+?
The cation H3O+ is known as the hydronium ion. If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium.
What is the hydronium ion used for?
The hydronium ion is used in various reactions and the production of different compounds. Both organic and inorganic chemistry includes hydronium ion to a large extent.
What is Lewis structure?
A lewis structure helps us to find out about the structure of the compound, types, and the number of bonds, physical properties, and how the compound interacts with other compounds. Drawing a lewis structure is pretty simple! There is a common way by which we can draw the lewis structure of any compound.
What is the concentration of H3O+?
Concentration of H3O+ is what we use to approximate the pH. pH is the negative logarithm to the base 10 of the hydronium ion concentration measured in mol/L. So you get H3O+ when you dissolve an Arrhenius acid in water.
What is the name of the ion that dissolves in water?
There’s H3O+ which is called the hydronium ion. That’s what you get when you dissolve an Arrhenius acid (a species that dissociates in water to form hydrogen ions, example: HCl) in water. The H+ from the acid combines with water to make H3O+.
Is phytate bad for you?
Some anions, such as phytate can be bad for you at sufficiently high concentrations while others like chloride are harmless. So you wouldn’t want to drink a solution of 0.1 M phytic acid. But I suspect that you are asking because you are wondering about the potential hazard of hydronium itself.
Is H3O+ a proton?
The hydronium ion, H3O^+, is a representation of a hydrated hydrogen ion (H^+ attached to a water molecule). The hydrogen ion is sometimes called a proton, but that would be correct only for the most common hydrogen ion, which, indeed, is simply a proton.
Is H3O+ an acid?
The hydronium ion itself is a very strong acid, the strongest that can exist in an aqueou. Continue Reading. There is no such thing as uncharged H3O, but if you really mean H3O+, not only can you drink it, you do so every day. It’s called the hydronium ion and is formed when an acid (specifically an Arrhenius acid) is added to water.
