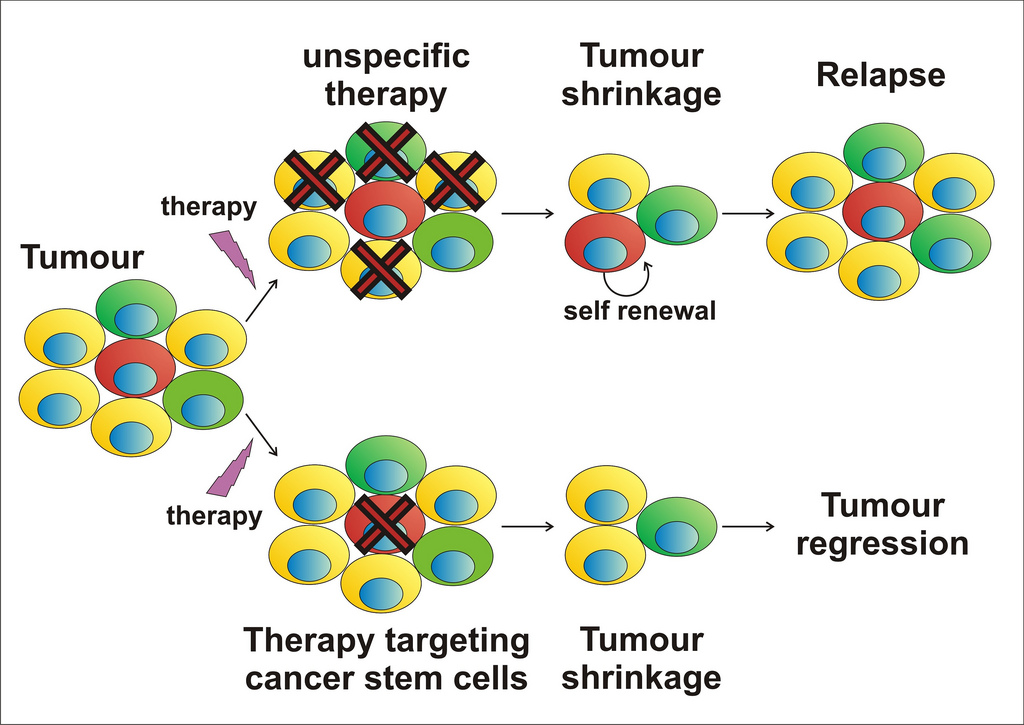
What diseases are treated with stem cells?
- Multiple Sclerosis. Multiple sclerosis is a chronic disease where inflammation is caused by the failure of the immune system, which damages cells of the brain or spinal cord.
- Cardiovascular Diseases. ...
- Diabetes and Diabetic Ulcer. ...
- Autism. ...
- Liver Diseases. ...
- Crohn’s Disease. ...
- Fibromyalgia. ...
- Parkinson’s Disease. ...
- Injuries and Musculoskeletal Pain. ...
- COVID-19. ...
What are the problems with stem cell therapy?
What you need to know:
- Blood and immunodeficiency conditions are the oldest known problems stem cell therapy can solve
- Here at Cellaxys, we do not treat these types of conditions
- Common conditions include: leukemia , lymphoma , anemia
What are the four types of stem cell therapy?
What are the four types of stem cells?
- Embryonic stem cells. Embryonic stem cells come from human embryos that are three to five days old.
- Non-embryonic (adult) stem cells.
- Induced pluripotent stem cells (iPSCs)
- Cord blood stem cells and amniotic fluid stem cells.
What are the benefits and issues with using stem cells?
- Abundant somatic cells of donor can be used
- Issues of histocompatibility with donor/recipient transplants can be avoided
- Very useful for drug development and developmental studies
- Information learned from the “reprogramming” process may be transferable for in vivo therapies to reprogram damaged or diseased cells/tissues
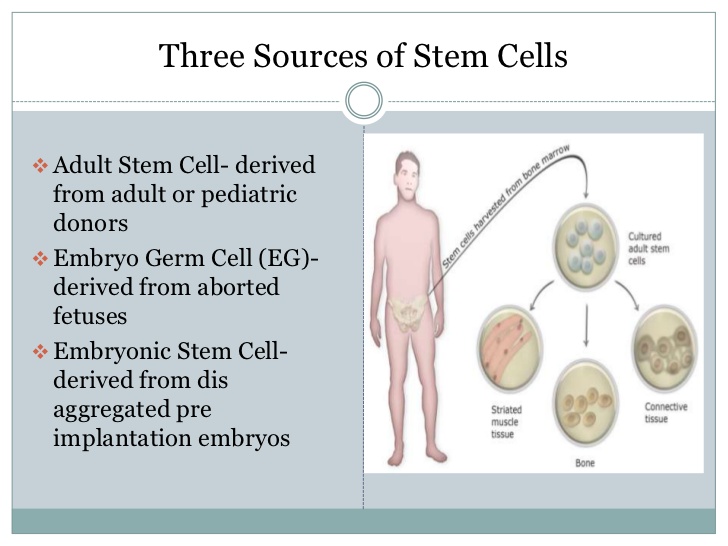
What is the process of stem cell therapy?
Blood stem cells are taken through a painless process called apheresis. Blood is taken from a vein and circulated through a machine that removes the stem cells and returns remaining blood and plasma back to the patient.Bone marrow stem cells are harvested from the donor in an operating room.
How long do stem cell treatments take?
This procedure isn't painful and is done while you're awake. It takes around 3 hours and may need to be repeated the next day if not enough cells are removed the first time.
Is stem cell treatment painful?
Stem cell treatments for back, knee, shoulder or joint pain serve as a perfect alternative to an invasive surgery that would require rehabilitation afterward. Our relatively painless procedure takes only 90 minutes and has far fewer risks and side effects than those associated with a complicated surgery.
What diseases can be treated with stem cells?
Diseases Treated with Stem Cell TransplantsAcute leukemia.Amegakaryocytosis or congenital thrombocytopenia.Aplastic anemia or refractory anemia.Chronic lymphocytic leukemia.Familial erythrophagocytic lymphohistiocytosis.Myelodysplastic syndrome of another myelodysplastic disorder.Osteopetrosis.More items...
What are the negative effects of stem cell therapy?
But unproven stem cell therapies can be particularly unsafe....Safety Concerns for Unproven Stem Cell TreatmentsAdministration site reactions,The ability of cells to move from placement sites and change into inappropriate cell types or multiply,Failure of cells to work as expected, and.The growth of tumors.
What is the cost of stem cell therapy?
The average cost of stem cell therapy ranges from under $5,000 to over $25,000, depending the type and sources of the stem cells, the patient's medical condition, and the number of treatments required.
Are you put to sleep for stem cell therapy?
Harvesting Stem Cells & Preparing for Injection We utilize light oral sedation to help relax the patient for the procedure. No general anesthetic is required.
Who is a good candidate for stem cell therapy?
If you suffer from painful disc or facet injury from overuse, trauma, or debilitating conditions like degenerative disc disease or spinal facet disease, you are likely an ideal candidate. Much of the early work in stem cell treatment for back pain has been devoted to chronic injuries.
Where do they inject stem cells?
Once the stem cells are harvested they are spun in a centrifuge to separate the cells that are then injected into the treatment area of the back, knee, shoulder, hip or other painful joints. The entire process last about 90 minutes and we only use stem cells taken from the patient.
What is the success rate of stem cell therapy?
The popularity of stem cell treatments has significantly increased, thanks to its high effectiveness and recorded success rates of up to 80%. It is a modern type of regenerative medical treatment that uses a unique biological component called stem cells.
What is the life expectancy after a stem cell transplant?
The relative mortality rate was high early after transplant as expected (standardized mortality ratio [SMR], 34.3 in the first 2-5 years) but persisted beyond 30 years (SMR, 5.4). Factors estimating mortality included age, high-risk disease, chronic GVHD, and use of PBSC grafts.
How long does it take to recover from stem cell transplant?
Recovery time depends on the type of transplant: Donated bone marrow or peripheral blood stem cell transplant can take 2-3 weeks. Cord blood engraftment can take 3-5 weeks. Self-donated stem cell transplant (autologous) takes about 10 days for recovery.
What is stem cell therapy?
Stem cell therapy is a non-invasive treatment that aims to replace damaged cells within the body. Mesenchymal stem cell therapy can be deployed systemically via IV or injected locally to target specific sites, depending on patient needs.
Why are stem cells important?
Studies have shown that stem cells can regenerate damaged or diseased tissues, reduce inflammation and modulate the immune system promoting better health and quality of life.
What is the capacity of a mesenchymal stem cell?
Mesenchymal stem cells (MSCs) also have the capacity to self-renew by dividing and developing into multiple specialized cell types present in a specific tissue or organ. Mesenchymal stem cells are adult stem cells, meaning they present no ethical concerns, MSCs are not sourced from embryonic material.
How do mesenchymal stem cells affect tissue repair?
Mesenchymal stem cells do this by influencing tissue repair via paracrine effects (cell signaling in order to change the behaviour of existing cells) or direct cell-to-cell contact. .
How is mesenchymal stem cell therapy deployed?
Mesenchymal stem cell therapy can be deployed systemically via IV or injected locally to target specific sites, depending on patient needs. Updated: July 14, 2021.
Which stem cell has the greatest proliferation rate?
Umbilical cord tissue-derived mesenchymal stem cells have the ability to differentiate into different cell types and have the greatest proliferation rate of the three mentioned types of stem cells (adipose, bone marrow, cord tissue). (7)
How do stem cells become different types of cells?
The process of stem cells maturing into new types of cells is called differentiation. This process is the most critical aspect of stem cell therapies, as the cells become the type of cells required for one’s body to heal.
What are the different types of stem cell therapy?
Let’s start by creating two categories of stem cell therapies – approved (by the FDA) and unapproved. Whether a stem cell therapy is approved or unapproved has critical implications for the science, effectiveness, and safety of the procedure.
How long does it take for stem cell therapy to work?
Furthermore, there is no proof that any stem cell therapy offered by stem cell clinics is effective or safe. Unlike FDA-approved procedures, which are subject to years of rigorous trials, unapproved treatments marketed directly to patients are developed and performed with little oversight.
What are the negative effects of stem cell therapy?
There are side effects associated with approved and unapproved stem cell therapies. The possible side effects of blood stem cell transplants are detailed on the Cancer.org website.
How long does stem cell therapy last?
In their advertising, stem cell clinics promise unsubstantiated relief or even cures for everything from knee pain to Parkinson’s disease, often taking advantage of vulnerable individuals who may feel they have nowhere else to turn.
Why are stem cells important?
Blood-forming stem cells are important because they grow into different types of blood cells. The main types of blood cells are: White blood cells, which are part of your immune system and help your body fight infection. Red blood cells, which carry oxygen throughout your body. Platelets, which help the blood clot.
What is stem cell transplant?
Stem cell transplants help restore blood-forming stem cells in people who have had theirs destroyed by certain cancer treatments. Stem cell transplants are procedures that restore blood-forming stem cells in people who have had theirs destroyed by the very high doses of chemotherapy or radiation therapy that are used to treat certain cancers.
What is the difference between autologous and allogeneic stem cells?
Transplants can be: Autologous, which means the stem cells come from you, the patient. Allogeneic, which means the stem cells come from someone else. The donor may be a blood relative but can also be someone who is not related. Syngeneic, which means the stem cells come from your identical twin, if you have one.
Why does graft versus tumor occur?
Graft-versus-tumor occurs when white blood cells from your donor (the graft) attack any cancer cells that remain in your body (the tumor) after high-dose treatments. This effect improves the success of the treatments.
How long does it take for your immune system to recover from a blood transplant?
Even after your blood counts return to normal, it takes much longer for your immune system to fully recover—several months for autologous transplants and 1 to 2 years for allogeneic or syngeneic transplants.
Where do you go to get an allogeneic stem cell transplant?
When you need an allogeneic stem cell transplant, you will need to go to a hospital that has a specialized transplant center. The National Marrow Donor Program® maintains a list of transplant centers in the United States. that can help you find a transplant center.
What type of cancer is stem cell transplant?
Who Receives Stem Cell Transplants. Stem cell transplants are most often used to help people with leukemia and lymphoma. They may also be used for neuroblastoma and multiple myeloma. Stem cell transplants for other types of cancer are being studied in clinical trials, which are research studies involving people.
How does stem cell therapy help?
Stem cell therapy can help patients to benefit from an increased range of motion in joints, stronger muscles, and better all-around flexibility. This is great news for those keen to return to an active daily routine. 3. Minimal recovery times.
Why do people use stem cells?
Sometimes, people suffer injuries so severe that parts of their body appear to be damaged beyond repair. In some of these cases, however, stem cell therapy could represent the key to recovery. This is because stem cells encourage damaged tissues to start regrowing, offering progressive pain relief and increased functionality of joints and muscles.
Why are biologics used in stem cell therapy?
This reduces the risk of body rejection compared to other types of treatments for chronic pain or injury.
What are the benefits of stem cells?
The benefits of stem cells are wide-ranging and include: 1. Effective pain reduction. Stem cell therapy helps to regenerate injured parts of the body and reduce inflammation, thereby offering pain relief that is long-lasting and treats the source of pain rather than simply masking it. 2.
How many types of stem cells are there?
There are four types of stem cell, including: Embryonic stem cells – these are present in embryos that are between three and five days old. Adult stem cells – these are found in human fat or bone marrow. Induced pluripotent stem cells – these are adult stem cells that have been altered so they operate more like embryonic stem cells.
Why are stem cells concentrated?
Sometimes, stem cells are concentrated to produce “specialized” functions, thereby helping to repair damaged heart cells, red blood cells, or brain cells . In many ways, stem cell treatment is still a budding field.
What is a stem cell?
What are stem cells? Stem cells (also known as “master cells”) are special human cells that can produce an indefinite number of “daughter cells”. These daughter cells will either function as new stem cells or as specialized cells such as muscle cells, brain cells, blood cells, or bone cells.
What is stem cell therapy?
Stem-cell therapy is the use of stem cells to treat or prevent a disease or condition. As of 2016. [update] , the only established therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone-marrow transplantation, but the cells can also be derived from umbilical cord blood.
Why are stem cells being studied?
Stem cells are being studied for a number of reasons. The molecules and exosomes released from stem cells are also being studied in an effort to make medications. In addition to the functions of the cells themselves, paracrine soluble factors produced by stem cells, known as the stem cell secretome, have been found to be another mechanism by which stem cell-based therapies mediate their effects in degenerative, autoimmune, and inflammatory diseases.
How many discrepancies were found in 2013 studies of autologous bone marrow stem cells on ventricular
In 2013, studies of autologous bone-marrow stem cells on ventricular function were found to contain "hundreds" of discrepancies. Critics report that of 48 reports, just five underlying trials seemed to be used, and that in many cases whether they were randomized or merely observational accepter-versus-rejecter, was contradictory between reports of the same trial. One pair of reports of identical baseline characteristics and final results, was presented in two publications as, respectively, a 578-patient randomized trial and as a 391-subject observational study. Other reports required (impossible) negative standard deviations in subsets of people, or contained fractional subjects, negative NYHA classes. Overall, many more people were reported as having receiving stem cells in trials, than the number of stem cells processed in the hospital's laboratory during that time. A university investigation, closed in 2012 without reporting, was reopened in July 2013.
How to regenerate tissue in adult?
A possible method for tissue regeneration in adults is to place adult stem cell "seeds" inside a tissue bed "soil" in a wound bed and allow the stem cells to stimulate differentiation in the tissue bed cells. This method elicits a regenerative response more similar to fetal wound-healing than adult scar tissue formation.
What are the effects of stem cells on animal models of brain degeneration?
Research has been conducted on the effects of stem cells on animal models of brain degeneration, such as in Parkinson's disease, Amyotrophic lateral sclerosis, and Alzheimer's disease. Preliminary studies related to multiple sclerosis have been conducted.
How do corneal stem cells help restore vision?
Since 2003, researchers have successfully transplanted corneal stem cells into damaged eyes to restore vision. "Sheets of retinal cells used by the team are harvested from aborted fetuses, which some people find objectionable." When these sheets are transplanted over the damaged cornea, the stem cells stimulate renewed repair, eventually restore vision. The latest such development was in June 2005, when researchers at the Queen Victoria Hospital of Sussex, England were able to restore the sight of forty people using the same technique. The group, led by Sheraz Daya, was able to successfully use adult stem cells obtained from the patient, a relative, or even a cadaver. Further rounds of trials are ongoing.
What is the FDA approved for?
The FDA has approved five hematopoietic stem-cell products derived from umbilical-cord blood, for the treatment of blood and immunological diseases. In 2014, the European Medicines Agency recommended approval of limbal stem cells for people with severe limbal stem cell deficiency due to burns in the eye.
What are stem cells?
Sometimes called the body’s “master cells,” stem cells are the cells that develop into blood, brain, bones, and all of the body’s organs. They have the potential to repair, restore, replace, and regenerate cells, and could possibly be used to treat many medical conditions and diseases. But the U.S. Food and Drug Administration is concerned ...
What is the FDA's response to stem cell products?
When stem cell products are used in unapproved ways— or when they are processed in ways that are more than minimally manipulated, which relates to the nature and degree of processing—the FDA may take (and has already taken) a variety of administrative and judicial actions, including criminal enforcement, depending on the violations involved.
Where do stem cells come from?
The FDA has the authority to regulate stem cell products in the United States. Today, doctors routinely use stem cells that come from bone marrow or blood in transplant procedures to treat patients with cancer and disorders of the blood and immune system. Electron micrograph of stem cells, color-enhanced for visual clarity.
Is stem cell treatment illegal?
Food and Drug Administration is concerned that some patients seeking cures and remedies are vulnerable to stem cell treatments that are illegal and potentially harmful. And the FDA is increasing its oversight and enforcement to protect people from dishonest and unscrupulous stem cell clinics, while continuing to encourage innovation so ...
Can stem cells be unsafe?
Please try again later. Researchers hope stem cells will one day be effective in the treatment of many medical conditions and diseases. But unproven stem cell treatments can be unsafe—so get all of the facts if you’re considering any treatment.
Does the FDA review stem cell products?
The FDA has reviewed many stem cell products for use in these studies. As part of the FDA’s review, investigators must show how each product will be manufactured so the FDA can make sure appropriate steps are being taken to help assure the product’s safety, purity, and strength (potency).
Is stem cell therapy FDA approved?
To do your part to stay safe, make sure that any stem cell treatment you are considering is either: FDA-approved, or; Being studied under an Investigational New Drug Application (IND), which is a clinical investigation plan submitted and allowed to proceed by the FDA. And see the boxed section below for more advice.