Will I be paid for clinical trials?
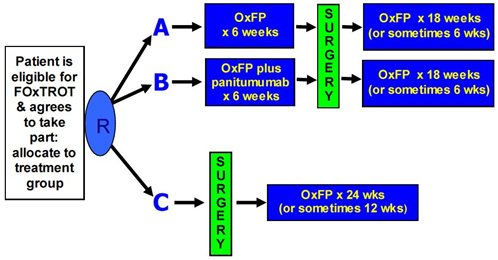
Arms, Groups, and Cohorts. In a clinical study, participants are often divided into groups. In interventional studies, participants are assigned to receive one or more interventions (or no intervention) so that researchers can evaluate the effects of the interventions on biomedical or health-related outcomes. Arm refers to each group or subgroup of participants in a clinical …
Are clinical trials in the real world?
Apr 22, 2015 · Abstract. Incorporating an emerging therapy as a new randomisation arm in a clinical trial that is open to recruitment would be desirable to researchers, regulators and patients to ensure that the trial remains current, new treatments are evaluated as quickly as possible, and the time and cost for determining optimal therapies is minimised.
What are the requirements for a clinical trial?
treatment arm Active arm Clinical trials In a placebo- controlled trial, the arm that receives a particular therapy
What is a single arm clinical trial?
An arm of a clinical trial is a group of patients receiving a specific treatment (or no treatment). Trials involving several arms, or randomized trials, treat randomly-selected groups of patients with different therapies in order to compare their medical outcomes. Experimental arms, which receive an experimental drug, are compared with control arms, which can receive an active comparator …
What are the different arms of a clinical trial?
Types of arms include experimental arm, active comparator arm, placebo comparator arm, sham comparator arm, and no intervention arm. Data collected at the beginning of a clinical study for all participants and for each arm or comparison group.
What is a 3 arm trial?
Abstract. A 3-arm trial design that includes an experimental treatment, an active reference treatment, and a placebo is useful for assessing the noninferiority of an experimental treatment.
What is a 4 arm study?
A Four Arm Study to Evaluate the Safety and Efficacy of 3 Different Doses of RVX-100 Versus Placebo in Subjects With Irritable Bowel Syndrome Accompanied by Diarrhea (IBS-D) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.Feb 26, 2010
What is the difference between an arm and a cohort?
Multiple arms allow the effects of different interventions to be compared. Participants in a control arm may receive no intervention, placebo, or an intervention with a known effect. In observational studies, a pre-defined population may be observed over time and is termed, a cohort.May 17, 2009
What is 2 arm randomized controlled trial?
Study design In trials with randomized and controlled design (e.g., a two-armed study with parallel groups), the effects of the study treatment (intervention) are compared with those of a control treatment and the patients are randomly assigned to the two groups.
What is a 2 arm study?
For a two-arm study, with two short-term outcomes, study participants are randomised to either the control or active intervention arms.Dec 9, 2019
What are the 4 phases of clinical trials?
Each stage of a clinical trial has its own purpose in ensuring that a treatment is safe and effective for use by the public.Phase 1 Clinical Trial. ... Phase 2 Clinical Trial. ... Phase 3 Clinical Trial. ... Monitoring Post-FDA Approval.Sep 2, 2021
How long is washout period?
A frequent recommendation is for the washout period to be at least 5 times the half-life of the treatment with the maximum half-life in the study. Endpoint evaluations can also be made at the end of a period to allow more time for the effects of prior treatments to dissipate.
What is a treatment arm?
Treatment Arm: A group or subgroup of participants in a clinical trial. Each group receives a specific intervention, study drug dose, or sometimes no intervention, according to the study protocol.
What are arms and interventions?
Arm refers to each group or subgroup of participants in a clinical trial that receives specfic interventions (or no intervention) according to the study protocol. This is decided before the trial begins.
How many arms can a clinical trial have?
The two arms in this case generally include the treatment arm and the control arm (alternative treatment/placebo arm). But RCTs can have more than two arms (multiple-armed RCT). One example would be a three-armed RCT comparing a treatment arm with an inactive control/placebo arm, and alternative active treatment.
What is clinical trial?
A clinical trial is a research study conducted with human beings to see if a new medical device, medication or other treatment is effective and safe. Some study participants will be assigned to a “control arm” or “control group” in the study. Those who are in the control arm will not receive the new medication, ...
How many children have autism?
Continue Learning about Medical Research. Autism, a developmental disorder that affects about one out of 68 U.S. children, typically involves communication and learning difficulties. It’s a sp.