Which monoclonal antibody is best?
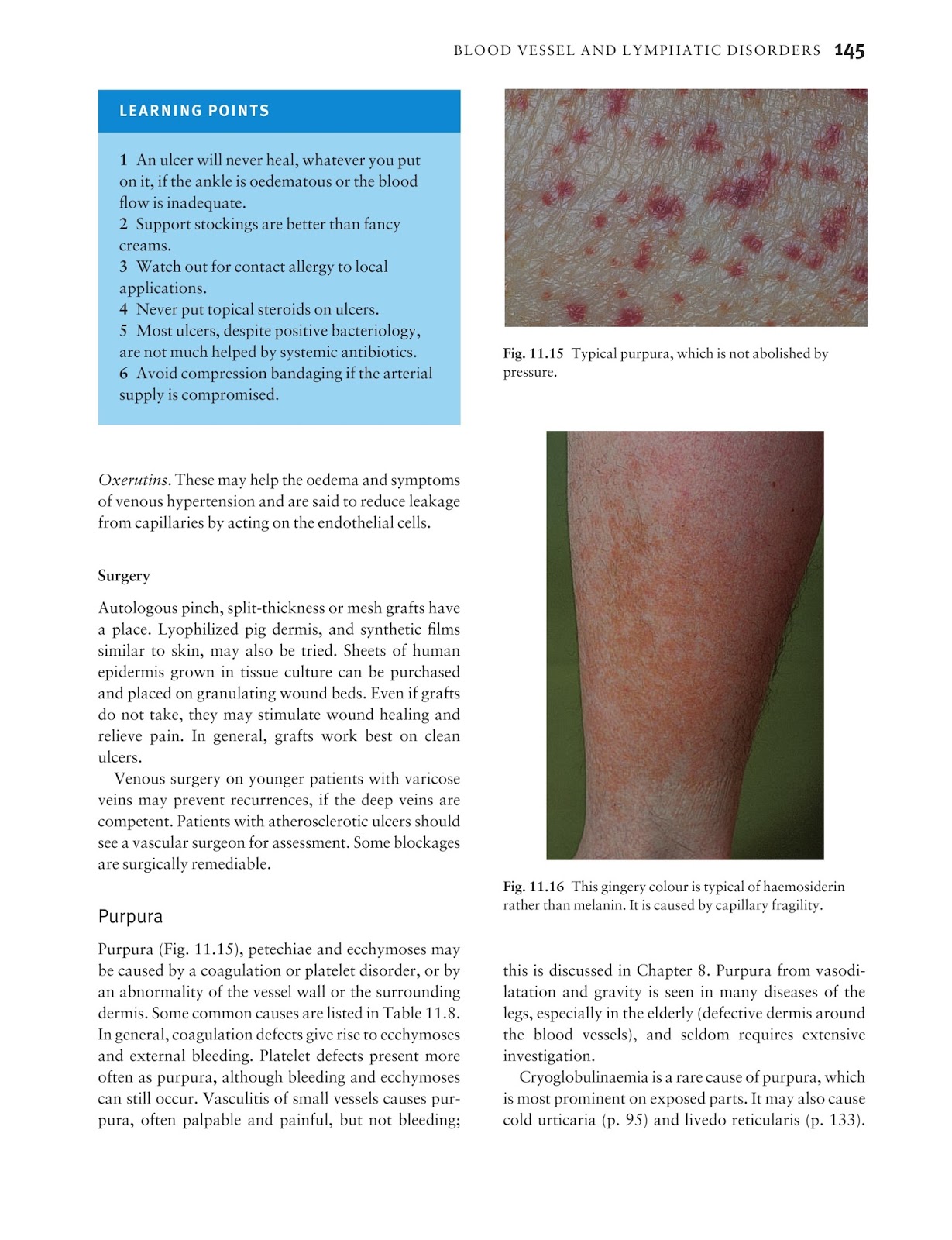
Feb 01, 2022 · Anti-SARS-CoV-2 Monoclonal Antibodies. The SARS-CoV-2 genome encodes 4 major structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N), as well as nonstructural and accessory proteins. The spike protein is further divided into 2 subunits, S1 and S2, that mediate host cell attachment and invasion.
What are the dangers of monoclonal antibodies?
What mAbs are made of. Monoclonal antibodies are man-made proteins that act like human antibodies in the immune system. There are 4 different ways they can be made and are named based on what they are made of. Murine: These are made from mouse proteins and the names of the treatments end in -omab.
What to expect from monoclonal antibody treatment?
testing in humans, and reviews of safety, efficacy, and quality. Monoclonal antibody treatment continues to be studied for its effectiveness and safety. Monoclonal antibody treatment is an additional way to help reduce the risk of hospitalization …
Are there side effects of monoclonal antibody treatment?
Consistent with existing payment methodologies for the care setting where you provide the treatment; For COVID-19 monoclonal antibody products administered before May 6, 2021, the Medicare payment rate is approximately $310. Medicare will establish codes and rates for administering new products as the FDA approves or authorizes each product.
How do monoclonal antibodies work against COVID-19?
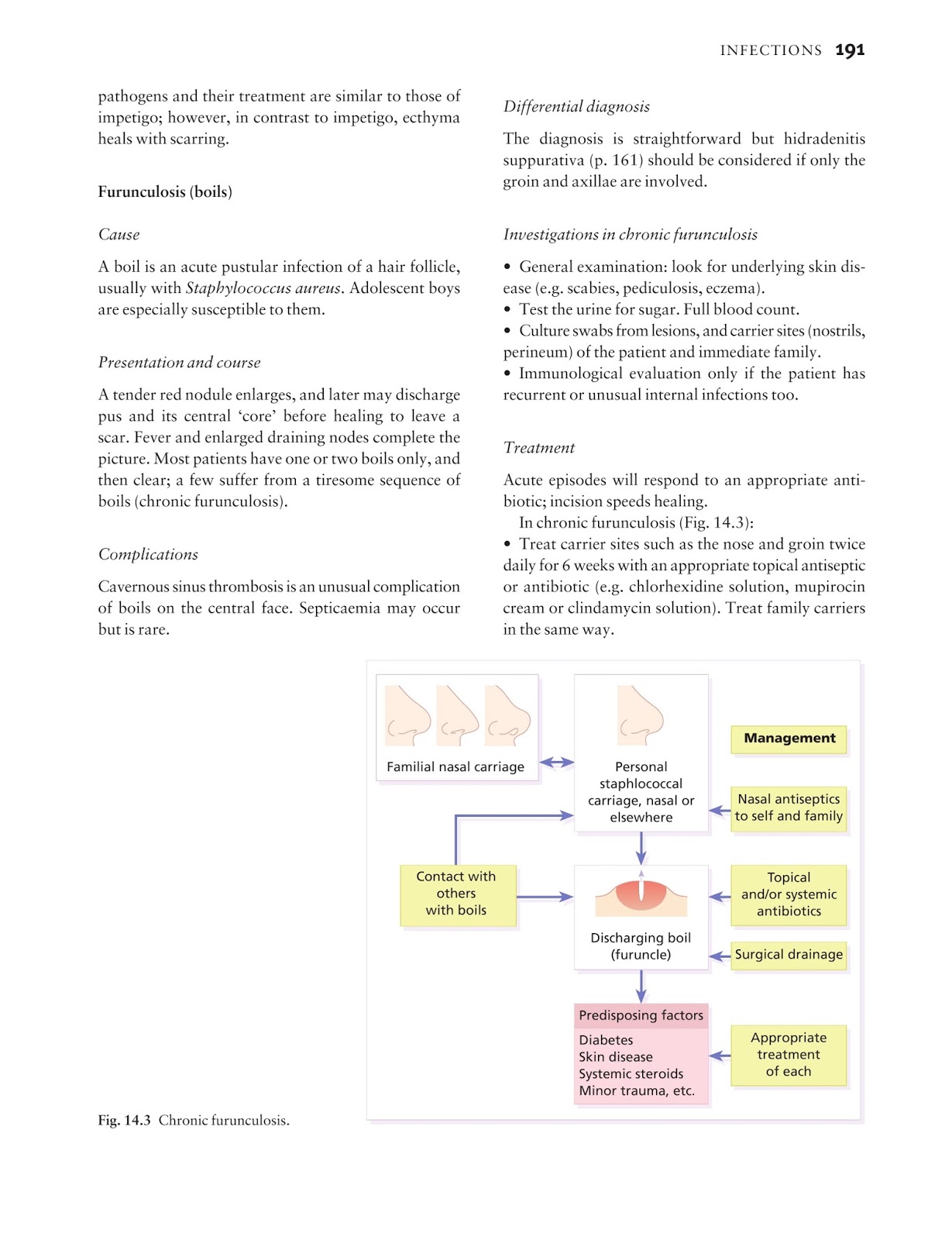
Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Is there a monoclonal antibody therapy for post COVID-19 exposure?
FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19 | FDA.Sep 16, 2021
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
What is the first drug that was approved by the FDA to treat COVID-19?
Remdesivir is the first drug approved by the FDA for treatment of hospitalized COVID patients over the age of 12.Jan 25, 2022
Which drug is approved by FDA to treat COVID-19?
Veklury (Remdesivir) is an antiviral drug approved for use in adults and pediatric patients [12 years of age and older and weighing at least 40 kilograms (about 88 pounds)] for the treatment of COVID-19 requiring hospitalization.Mar 31, 2022
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
Who could benefit from monoclonal antibody therapy to prevent COVID-19?
See full answerVaccines are the best way to protect against COVID-19. But some people with weakened immune systems do not produce enough antibodies after vaccination, and others are severely allergic to the vaccine. The FDA recently authorized Evusheld, a pre-exposure prophylaxis (PrEP) monoclonal antibody therapy developed by AstraZeneca, which should help prevent COVID-19 in these populations.To be eligible for Evusheld, individuals must be 12 years or older and have a moderately to severely weakened immune system, or have a history of severe adverse reactions to the COVID-19 vaccine or its components. In addition, the therapy cannot be given to someone with a current SARS-CoV-2 infection, or who has been recently exposed to someone who is infected. Evusheld is given as two consecutive shots, and evidence suggests it can help prevent symptomatic infection for at least six months.Apr 1, 2022
Is there an antibody cocktail for COVID-19?
The treatment, bamlanivimab and etesevimab administered together, was granted FDA emergency use authorization in February. Eli Lilly and the FDA stipulated that the antibody cocktail is authorized as a COVID-19 prophylaxis only for individuals who have been exposed to the virus.Sep 16, 2021
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
Can I get COVID-19 again after having the vaccine?
Getting COVID-19 after you've been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you.Nov 10, 2021
Who should not take the Pfizer-BioNTech COVID-19 vaccine?
If you have had a severe allergic reaction to any ingredient in the Pfizer-BioNTech COVID-19 vaccine (such as polyethylene glycol), you should not get this vaccine. If you had a severe allergic reaction after getting a dose of the Pfizer-BioNTech COVID-19 vaccine, you should not get another dose of an mRNA vaccine.
Naked Monoclonal Antibodies
Naked mAbs are antibodies that work by themselves. There is no drug or radioactive material attached to them. These are the most common type of mAb...
Conjugated Monoclonal Antibodies
Monoclonal antibodies (mAbs) joined to a chemotherapy drug or to a radioactive particle are called conjugated monoclonal antibodies. The mAb is use...
Bispecific Monoclonal Antibodies
These drugs are made up of parts of 2 different mAbs, meaning they can attach to 2 different proteins at the same time. An example is blinatumomab...
Possible Side Effects of Monoclonal Antibodies
Monoclonal antibodies are given intravenously (injected into a vein). The antibodies themselves are proteins, so giving them can sometimes cause so...
What is conjugated monoclonal antibody?
Conjugated monoclonal antibodies. Conjugated mAbs are combined with a chemotherapy drug or a radioactive particle. These mAbs are used as a homing device to take one of these substances directly to the cancer cells. The mAb circulates throughout the body until it can find and hook onto the target antigen.
What is the purpose of monoclonal antibodies?
These are known as monoclonal antibodies (mAbs or Moabs). Monoclonal antibodies are used to treat many diseases, including some types of cancer. To make a monoclonal antibody, researchers first have to identify the right antigen to attack.
What are the side effects of mAbs?
It can cause side effects such as high blood pressure, bleeding, poor wound healing, blood clots, and kidney damage.
How do naked mAbs work?
(See Immune Checkpoint Inhibitors and Their Side Effects .) Other naked mAbs work mainly by attaching to and blocking antigens on cancer cells (or other nearby cells) that help cancer cells grow or spread.
What is an antibody?
An antibody is a protein that sticks to a specific protein called an antigen. Antibodies circulate throughout the body until they find and attach to the antigen. Once attached, they can force other parts of the immune system to destroy the cells containing the antigen. Researchers can design antibodies that specifically target a certain antigen, ...
Is ibritumomab tiuxetan radioactive?
Ibritumomab tiuxetan (Zevalin) is an example of a radiolabeled mAb. This is an antibody against the CD20 antigen, which is found on lymphocytes called B cells. The antibody delivers radioactivity directly to cancer cells. It is made of both an mAb drug (rituximab) and a radioactive substance (Yttrium-90).
What are mAbs made of?
There are 4 different ways they can be made and are named based on what they are made of. Murine: These are made from mouse proteins and the names of the treatments end in -omab.
When is bamlanivimab approved for use?
On November 9, 2020 , the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for the investigational monoclonal antibody therapy, bamlanivimab, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients with positive COVID-19 test results who are at high risk for progressing to severe COVID-19 and/or hospitalization. Bamlanivimab may only be administered in settings in which health care providers have immediate access to medications to treat a severe infusion reaction, such as anaphylaxis, and the ability to activate the emergency medical system (EMS), as necessary. Review the Fact Sheet for Health Care Providers EUA of Bamlanivimab regarding the limitations of authorized use.
Can you use bamlanivimab in emergency settings?
Bamlanivimab may only be administered in settings in which health care providers have immediate access to medications to treat a severe infusion reaction, such as anaphylaxis, and the ability to activate the emergency medical system (EMS), as necessary.
What is monoclonal antibody?
Monoclonal antibodies are immune system proteins that are created in the lab. Antibodies are produced naturally by your body and help the immune system recognize germs that cause disease, such as bacteria and viruses, and mark them for destruction.
Why are monoclonal antibodies used in immunotherapy?
Some monoclonal antibodies are also immunotherapy because they help turn the immune system against cancer. For example, some monoclonal antibodies mark cancer cells so that the immune system will better recognize and destroy them.
What antibodies kill cancer cells?
Other monoclonal antibodies bring T cells close to cancer cells, helping the immune cells kill the cancer cells. An example is blinatumomab (Blincyto®), which binds to both CD19, a protein found on the surface of leukemia cells, and CD3, a protein on the surface of T cells. This process helps the T cells get close enough to ...
Can monoclonal antibodies cause side effects?
Monoclonal antibodies can cause side effects, which can differ from person to person. The ones you may have and how they make you feel will depend on many factors, such as how healthy you are before treatment, your type of cancer, how advanced it is, the type of monoclonal antibody you are receiving, and the dose.
What is the function of antibodies?
Antibodies are proteins that exist in our bodies as part of our immune system to recognize and defend against harmful viruses and bacteria. Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
Does infusion cause nausea?
Some people may experience infusion-related side effects, such as nausea and dizziness, that are short-lived and go away on their own. As with any medication, there is the potential for mild or more severe allergic reactions, which are uncommon.