Medication
As not everyone with Alzheimer’s will experience the disease the same way, treatment plans might look different as well. Although there is no cure right now, finding a cure for Alzheimer’s disease and a treatment that stops disease progression is an active area of biomedical research. Available treatments for Alzheimer's
Self-care
You may use the terms Alzheimer’s and dementia as if they mean the same thing, but they’re really two different terms. Here’s the difference. Dementia is a syndrome linked to problems with remembering, learning new things, focusing and making decisions that affect everyday life.
See more
Although Alzheimer's disease typically affects adults 65 years and older, early onset AD is when symptoms begin before 65, typically in your 40s and 50s .
Is there a cure for Alzheimer's?
Dementia and Alzheimer's: What Are the Differences?
- Dementia vs. Alzheimer’s. ...
- Dementia. Dementia is a syndrome, not a disease. ...
- Alzheimer’s disease. Dementia is the term applied to a group of symptoms that negatively impact memory, but Alzheimer’s is a progressive disease of the brain that slowly causes impairment in ...
- Alzheimer’s vs. ...
- Treating dementia vs. ...
- Outlook for people with dementia vs. ...
Is dementia and Alzheimer's the same thing?
When was Alzheimer's symptoms start before age 65?
What is the difference between dementia and Alzheimers?
What are the current treatments for Alzheimer's disease?
Aducanumab is the only disease-modifying medication currently approved to treat Alzheimer's. This medication is a human antibody, or immunotherapy, that targets the protein beta-amyloid and helps to reduce amyloid plaques, which are brain lesions associated with Alzheimer's.
What are 3 treatments for Alzheimer's?
Three cholinesterase inhibitors are commonly prescribed:Donepezil (Aricept) is approved to treat all stages of the disease. It's taken once a day as a pill.Galantamine (Razadyne) is approved to treat mild to moderate Alzheimer's. ... Rivastigmine (Exelon) is approved for mild to moderate Alzheimer's disease.
Is there a cure for Alzheimer's 2021?
On June 7, 2021, the FDA granted accelerated approval to aducanumab (brand name Aduhelm), the first drug in 18 years for Alzheimer's disease.
What is the new Alzheimer's drug that was just approved?
Aduhelm is a monoclonal antibody treatment delivered as an infusion. The drug targets amyloid, a protein that clumps in the brains of Alzheimer's patients. While Aduhelm does reduce amyloid plaques in patients' brains, so do many other medications that haven't been shown to slow cognitive decline.
Is there medication to slow down Alzheimer's?
There are no drug treatments that can cure Alzheimer's disease or any other common type of dementia. However, there are medicines for Alzheimer's disease that can ease symptoms for a while, or slow down their progression, in some people.
What is the best treatment for dementia?
Cognitive stimulation therapy It is currently the only psychological dementia treatment directly recommended by the National Institute for Health and Care Excellence (NICE) to help people with mild or moderate dementia.
When will aducanumab be available to patients?
In June 2021, the Food and Drugs Administration (FDA) - the drug regulatory body in the USA - approved Aducanumab for clinical use in people with Alzheimer's disease in the USA.
What will aducanumab cost?
Background and Objectives Aducanumab was granted accelerated approval with a conflicting evidence base, near-unanimous Food and Drug Administration Advisory Committee vote to reject approval, and a widely criticized launch price of $56,000 per year. The objective of this analysis was to estimate its cost-effectiveness.
How effective is aducanumab for Alzheimer's?
Clinical trials studying the effectiveness of aducanumab did not prove that the drug improved cognition. In fact, two trials were stopped early because no clinical benefit was shown. Biogen reviewed the data again and noted a small improvement of symptoms in one subgroup of patients.
Will be a new drug for dementia by 2020?
Aducanumab (Aduhelm™) has received accelerated approval as a treatment for Alzheimer's disease from the U.S. Food and Drug Administration (FDA). This is the first FDA-approved therapy to address the underlying biology of Alzheimer's disease.
Will Medicare cover new Alzheimer's drug?
April 8, 2022 -- Federal officials have made their final decision: Medicare will only pay for patients to get the new Alzheimer's drug aducanumab (Aduhelm) if the patients are participating in clinical trials.
What are the risks of aducanumab?
Like all drugs, aducanumab can come with side effects. The most common observed during clinical trials were brain swelling and tiny brain bleeds. Headaches, falls, diarrhea and confusion were also reported.
What is the drug used to treat Alzheimer's disease?
Researchers are studying ways to treat inflammatory processes at work in Alzheimer's disease. The drug sargramostim (Leukine) is currently in research. It's thought that the drug may stimulate the immune system to protect the brain from harmful proteins.
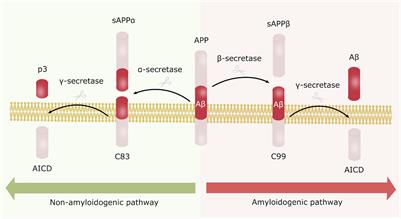
What are the plaques in Alzheimer's?
Plaques are a characteristic sign of Alzheimer's disease. Strategies aimed at beta-amyloid include: Recruiting the immune system. Several drugs — known as monoclonal antibodies — may prevent beta-amyloid from clumping ...
What is the best way to reduce beta-amyloid?
Production blockers. These therapies may reduce the amount of beta-amyloid formed in the brain. Research has shown that beta-amyloid is produced from a "parent protein" in two steps performed by different enzymes. Several experimental drugs aim to block the activity of these enzymes.
Is dementia related to heart disease?
Growing evidence suggests that brain health is closely linked to heart and blood vessel health. The risk of developing dementia appears to increase as a result of many conditions that damage the heart or arteries. These include high blood pressure, heart disease, stroke, diabetes and high cholesterol.
Does beta secretase slow cognitive decline?
They're known as beta- and gamma-secretase inhibitors. Recent studies showed that the beta-secretase inhibitors did not slow down cognitive decline and were associated with significant side effects in those with mild or moderate Alzheimer's, which has decreased enthusiasm for this mechanism of drug.
Does Alzheimer's disease stop memory loss?
These Alzheimer's treatments boost performance of chemicals in the brain that carry information from one brain cell to another. However, these treatments don't stop the underlying decline and death ...
Is lecanemab approved by the FDA?
Experts also need to identify which patients may benefit from the drug. The monoclonal antibody lecanemab shows promise in removing amyloid and has moved into phase 3 clinical trials.
What is the best treatment for Alzheimer's?
Aducanumab is the only disease-modifying medication currently approved to treat Alzheimer’s. This medication is a human antibody, or immunotherapy, that targets the protein beta-amyloid and helps to reduce amyloid plaques, which are brain lesions associated with Alzheimer’s.
What are the interventions for Alzheimer's?
In ongoing clinical trials, scientists are developing and testing several possible interventions, including immunization therapy, drug therapies, cognitive training, physical activity, and treatments for cardiovascular disease and diabetes.
Why was aducanumab approved?
The approval of aducanumab was based on the ability of the drug to reduce amyloid in the brain. When using the accelerated approval pathway, drug companies are required to conduct additional studies to determine whether there is in fact clinical benefit after the drug is approved.
How does memantine help Alzheimer's patients?
For example, memantine may help a person in the later stages of the disease maintain his or her ability to use the bathroom independently for several more months, a benefit for both the person with Alzheimer's and caregivers. Memantine is believed to work by regulating glutamate, an important brain chemical.
What is the FDA's Accelerated Approval Program?
FDA’s Accelerated Approval Program. Aducanumab was approved through the FDA’s Accelerated Approval Program, which provides a path for earlier approval of drugs that treat certain serious conditions. This helps people living with the disease gain earlier access to the treatment.
What is the National Institute on Aging's ADEAR Center?
The National Institute on Aging’s ADEAR Center offers information and free print publications about Alzheimer’s disease and related dementias for families, caregivers, and health professionals. ADEAR Center staff answer telephone, email, and written requests and make referrals to local and national resources.
What tests are needed for aducanumab?
Before prescribing aducanumab, doctors may require PET scans or an analysis of cerebrospinal fluid to evaluate whether amyloid deposits are present in the brain. This can help doctors make an accurate diagnosis of Alzheimer’s before prescribing the medication.
What is a guide to quality care from the perspectives of people living with dementia?
A Guide to Quality Care from the Perspectives of People Living with Dementia offers insights into how people living with dementia view quality care and what they want from care providers and caregivers.
What is a practice recommendation?
They are intended for professional care providers who work with individuals living with dementia and their families in long-term and community-based care settings. The Practice Recommendations are published as a supplement to The Gerontologist.
Guidelines Summary
In 2018, the Alzheimer's Association released the first clinical practice guidelines for the evaluation of cognitive impairment suspected to be a result of Alzheimer's disease and related dementias in both primary care and specialty care settings. The guidelines contain 20 recommendations, 16 of which are classified as "A" recommendations.
Alzheimer's Association
In 2018, the Alzheimer's Association released the first clinical practice guidelines for the evaluation of cognitive impairment suspected to be a result of Alzheimer's disease and related dementias in both primary care and specialty care settings. The guidelines contain 20 recommendations, 16 of which are classified as "A" recommendations.
What are the challenges associated with pharmacological treatment of dementia?
The pharmacological treatment of dementia is associated with important challenges such as complexities in the clinical presentation and diagnosis, non-availability of therapeutic agents with robust effectiveness and issues related to tolerability of medications used in the treatment of dementia.
What is the severity of dementia?
The overall severity of the dementia is best expressed as the level of decline in memory or other cognitiveabilities, whichever is the more severe (e.g. mild decline in memory and moderate decline in cognitive abilitiesindicate a dementia of moderate severity). G2.
What is Parkinson disease dementia?
The term Parkinson disease dementia (PDD) should be used to describe dementia that occurs in the context of well-established Parkinson disease. In a practice setting the term that is most appropriate to the clinical situation should be used and generic terms such as Lewy body disease are often helpful.
What is dementia syndrome?
DEMENTIA SYNDROME. Dementia is a syndrome due to disease of the brain, usually chronic, characterized by a progressive, global deterioration in intellect including memory, learning, orientation, language, comprehension and judgment. It mainly affects older people, after the age of 65 years.
Is delirium a risk factor for dementia?
Delirium in late life is often superimposed on pre-existing dementia and can be the reason for help seeking. Dementia is the leading risk factor for delirium in an older person. Occurrence of delirium in turn is a risk factor for subsequent dementia in older people without pre-existing dementia.
Is there evidence for dementia?
There is no evidence from the history, physical examination or special investigations for any other possible cause of dementia (e.g. cerebrovascular disease, Parkinson's disease, Huntington's disease, normal pressure hydrocephalus), a sysytemic disorder (e.g. hypothyroidism, vit.
Is memantine a good treatment for dementia?
Memantine has been approved for moderate to severe Alzheimer's dementia. Combination of Donepezil and Memantine has been approved for moderate to Severe Alzheimer's dementia. Rivastigmine Transdermal patch (4.6mg, 9.5mg or 13,3mg per 24 hours) has been approved for mild to moderate Alzheimer's dementia.