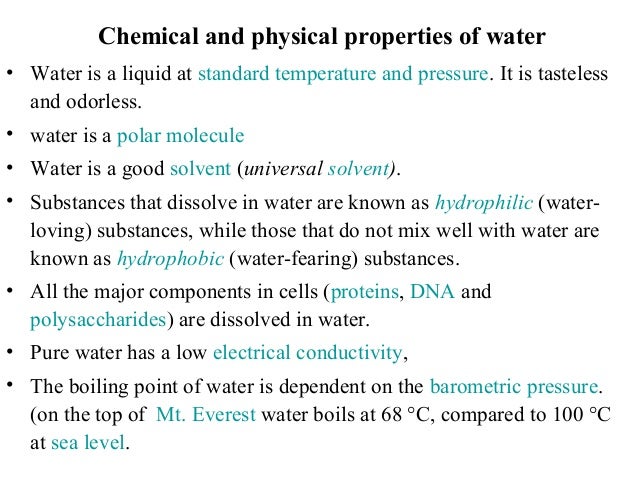
Conductivity measurements are useful to indicate the amount of dissolved ions present in a water sample and can serve as a measure of water quality. The measurement of conductivity in water can help to identify the concentration of solutions, the purity of the water, as well as the detection of contaminants.
How to test water conductivity?
Conductivity 1. Aim a. To determine electrical conductivity of tap water b. To study the effect of different types of dissolved salts and their concentration on electrical conductivity of water 2. Method a. Read SAP for measurement of EC. Familiarise yourself with the operation of the EC meter available in the laboratory.
How to calibrate the conductivity probe?
What Is the Standard Method for Calibration of a Conductivity Meter?
- Conductivity and Meter Measurement. A conductivity meter measures an aqueous solution's ability to transmit an electrical current. ...
- Preparing to Calibrate. ...
- Calibrating Conductivity. ...
- Taking Measurements and Maintenance. ...
What causes conductivity in water to increase?
Significant changes (usually increases) in conductivity may indicate that a discharge or some other source of disturbance has decreased the relative condition or health of the water body and its associated biota. Generally, human disturbance tends to increase the amount of dissolved solids entering waters which results in increased conductivity.
How to test for conductivity?
Tips for accurately measuring conductivity, resistivity, TDS and salinity
- Use a suitable conductivity sensor. ...
- Understand and anticipate the effects of temperature. ...
- Accurately use the temperature compensation (TC) function. ...
- Carefully set the temperature compensation settings. ...
- Take a conductivity reading only after temperature equilibrium is achieved. ...
- Minimize the use of elaborate multi-point calibrations. ...
Why is conductivity important in water quality testing?
Why Conductivity Is Important in Purified Water Quality Testing. Conductivity measures water’s ability to conduct electricity due to the presence or absence of certain ions. While pure water conducts electricity poorly, water that has certain chemicals or elements in it, and at varying amounts—including sodium, magnesium, calcium, ...
Why is water conductivity important?
Water conductivity plays an important role in ensuring water quality. Learn more about the different kinds of testing equipment that can be used to measure water conductivity here.
What is the difference between purified water and purified water?
1. Purified water. Purified water has been stripped of all or most of its impurities. Purified water can still have some impurities in it.
What is ultrapure water?
Ultrapure water. Ultrapure water, like the name suggests, has been purified to an excruciating level. This water is commonly used in industries that have extremely high standards of cleanliness and water purity, including the semiconductor, pharmaceutical, and solar photovoltaic industries. It’s also used extensively in experiments ...
Why is purified water used in labs?
In lab settings, water conductivity needs to be measured because researchers and scientists need to know the exact composition of the water they’re experimenting with in order to get accurate, repeatable results. This is why purified water is so common in the pharmaceutical, semiconductor, and solar industries.
How is water purified?
Water can be purified through many different processes, including distillation, carbon filtration, deionization, and reverse osmosis. 2. Distilled water. Distilled water has been boiled in order to have contaminants removed. As water is boiled, it is captured as steam, and minerals and other compounds are left behind.
What is a handheld water test?
Handheld Testers are meters that can be dropped into water to measure several characteristics, including conductivity, salinity, temperature, and pH levels, among other things. Portables are water quality measurement meters that are perfect for field research applications. Researchers in the environmental sector use these devices ...
What is conductivity sensor?
More often than not, conductivity sensors are the workhorses of water quality instrumentation. They are more stabile, more robust, and longer lasting than pH, ORP, dissolved oxygen or other measurements. Every so often, however, they will produce erratic readings.
What causes caustic corrosion?
Caustic Corrosion. High caustic (NaOH) concentration can result from porous metal oxide deposits on tube metal surfaces or steam blanketing. Steam blanketing allows salts to concentrate on boiler metal surfaces. A steam layer forms between the boiler water and the tube wall, causing an inefficient heat transfer.
What causes increased conductivity in water?
Generally, human disturbance tends to increase the amount of dissolved solids entering waters which results in increased conductivity. Water bodies with elevated conductivity may have other impaired or altered indicators as well.
Why is conductivity important?
Conductivity is useful as a general measure of water quality. Each water body tends to have a relatively constant range of conductivity that, once established, can be used as a baseline for comparison with regular conductivity measurements. Significant changes in conductivity could then be an indicator that a discharge or some other source ...
What does it mean when a discharge has a significant change in conductivity?
Significant changes (usually increases) in conductivity may indicate that a discharge or some other source of disturbance has decreased the relative condition or health of the water body and its associated biota.
Why does salt have low conductivity?
Because dissolved salts and other inorganic chemicals conduct electrical current, conductivity increases as salinity increases. Organic compounds like oil do not conduct electrical current very well and therefore have a low conductivity when in water. Conductivity is also affected by temperature: the warmer the water, the higher the conductivity.
