Full Answer
What is nitrification in wastewater treatment?
Nitrification is one of the two primary mechanisms for ammonia removal in aerobic wastewater systems. Nitrification is a two-step process performed by two categories of bacteria, ammonia oxidizers and nitrite oxidizers. Ammonia oxidizing bacteria (AOB) convert ammonia to nitrite through the ammonia oxidation pathway.
What is biodegradable nitrification?
Biological nitrification is the microbe-mediated process of oxidizing ammonia to remove nitrogenous compounds from wastewaters. Domestic sewage typically contains 20 to 40 mg/L (ppm) of ammonia nitrogen (NH. 4. -N). Organic matter containing nitrogen, e.g., protein and nucleic acid, also biodegrades to release ammonia.
How long does it take for nitrifier to work?
Typically, an HRT of greater than 10 hours is recommended for nitrification and a greater than 8 days MCRT is considered ideal for maintaining an adequate nitrifier population. This often takes more time in systems with adverse conditions, which leads operators to increase their MCRT during adverse conditions such as low temperature.
How long does it take for nitrifiers to remove ammonia?
Ammonia is converted to nitrite, then nitrate. As this test uses a tap water-based media without carbon and heterotrophic growth, pH variation tends to lead to slow growth of nitrifiers, and nitrite oxidizers. This testing confirmed that after 6 months in refrigeration, nitrifiers were still capable of removing ammonia in a solution.

How does nitrification happen in wastewater treatment?
One treatment system used for denitrifying wastewater effluent is the denitrifying filter. In addition to the reduction of total nitrogen, this treatment process removes suspended solids from the effluent. Nitrification is a microbial process by which ammonia is sequentially oxidized to nitrite and then to nitrate.
How long does it take for denitrification to occur?
Denitrification can occur in aerobic (adequate oxygen) conditions, but to a relatively insignificant degree. Wet soils are generally the trigger for denitrification to oc- cur. Nitrogen gases can begin to appear as soon as 15 minutes after saturation if conditions are favorable.
How do you speed up nitrification?
Filter media is one of the best habitats for Nitrifying bacteria; it receives constant water flow, little light, and is very porous. It may seem counterproductive, but letting your filter run during the cycle can help speed up the process greatly (especially if you can get a filter from a mature tank!).
What are the 5 stages of wastewater treatment?
Treatment StepsStep 1: Screening and Pumping. ... Step 2: Grit Removal. ... Step 3: Primary Settling. ... Step 4: Aeration / Activated Sludge. ... Step 5: Secondary Settling. ... Step 8: Oxygen Uptake. ... Sludge Treatment.
At what temperature does nitrification start?
The optimum temperature for the growth of nitrifying bacteria, according to the literature, is between 28° C and 36° C, although an optimum temperature of up to 42° C has been reported for Nitrobacter by Painter (1970).
What happens if denitrification does not occur?
Denitrification causes nitrites and nitrates to be converted into atmospheric nitrogen. In the absence of denitrification, nitrogen is not returned to the atmosphere, hence is contained and not recycled.
How long does it take for nitrites to turn into nitrate?
This process normally takes anywhere from 2-6 weeks. At temperatures below 70F, it takes even longer to cycle a tank. In comparison to other types of bacteria, Nitrifying bacteria grow slowly.
How long does it take for ammonia to turn into nitrate?
Beneficial bacteria is needed to take toxic fish waste called ammonia and convert it into nitrite and nitrate. Growing this beneficial bacteria takes time! It may take 4 to 6 weeks for the process to complete.
How long does a nitrogen cycle take?
Even with the addition of bacteria supplements, the nitrogen cycle can take between six and seven weeks to complete and stabilize.
What are the 7 steps in wastewater treatment?
The Wastewater Treatment ProcessStage One — Bar Screening. ... Stage Two — Screening. ... Stage Three — Primary Clarifier. ... Stage Four — Aeration. ... Stage Five — Secondary Clarifier. ... Stage Six — Chlorination (Disinfection) ... Stage Seven — Water Analysis & Testing. ... Stage Eight — Effluent Disposal.
What are the 3 stages of wastewater treatment?
There are three main stages of the wastewater treatment process, aptly known as primary, secondary and tertiary water treatment.
What is the final step of water treatment?
Disinfection. The final stage in the community water treatment process involves adding a disinfectant such as chlorine or chloramine to the water supply. Chlorine has been used since the late 1800s. The type of chlorine used in water treatment is monochloramine.
What is nitrification in wastewater?
Nitrification is one of the two primary mechanisms for ammonia removal in aerobic wastewater systems. Nitrification is a two-step process performed by two categories of bacteria, ammonia oxidizers and nitrite oxidizers. Ammonia oxidizing bacteria (AOB) convert ammonia to nitrite through the ammonia oxidation pathway.
How much of the population of nitrifying bacteria is activated sludge?
Nitrifying bacteria make up 4-6% of the population in an activated sludge process and have limited diversity compared to heterotrophic bacteria in wastewater. This makes them more susceptible to toxicity.
How long do nitrifiers stay in the refrigerator?
Testing, in this case, was run on nitrifiers that were stored in the refrigerator for approximately 6 months after growth in order to determine if nitrifiers remain active after this period. Nitrifier reactors were run in triplicate, while control reactors (no nitrifiers) were run in replicate.
Why are nitrifiers needed?
Nitrifiers require specific operating conditions to function effectively and are more sensitive to toxicity and other adverse conditions than most wastewater heterotrophs. If optimal conditions are present in a wastewater plant, nitrifier populations will be more robust and less susceptible to upsets.
What temperature do nitrifiers need to grow?
Nitrifiers prefer a temperature range between 15–30°C. Nitrifiers have trouble growing fast enough to maintain a population below 15°C, and sometimes have problems with low dissolved oxygen levels above 30°C as oxygen solubility decreases in higher temperatures. Nitrifiers have been recorded functioning effectively outside this temperature range as the bacterial population is often able to acclimate to varied temperatures. Rapid temperature changes also have significant adverse effects on nitrification since nitrifiers are unable to adjust quickly due to their slow growth rates. Nitrifiers have been observed functioning over 35°C (as high as 52°C) in a recent study (Knight, 2019), which had been thought to be near impossible.
What is the primary form of ammonia removal?
Nitrification becomes the primary form of ammonia removal after levels of BOD have been exhausted. Ammonia is often released in the decomposition of urea but will also appear in lower quantities due to the degradation of proteins and other nitrogen-containing molecules. Organic forms of nitrogen, such as amino acids and proteins, tend to be favored forms of nitrogen for heterotrophic bacteria. This means in wastewater treatment systems some excess ammonia is often present that must be oxidized by nitrifiers to achieve effluent limits for ammonia. In cases of lost nitrification , this extra ammonia is what is observed in waste discharge.
Why is alkalinity important for nitrification?
The first is nitrifiers use dissolved carbon dioxide, carbonate, and bicarbonate as their carbon source for autotrophic production of glucose, and (CO2, HCO3-, CO3-2) are major contribu tors to a system’s alkalinity. The second reason is nitrifiers produce nitric acid during ammonia oxidation and if alkalinity is low, pH fluctuations due to acid production can lead to poor nitrifier growth. This means in cases of low alkalinity, it is much more beneficial to supplement alkalinity with carbonate or bicarbonate, rather than other commonly added basic compounds such as sodium hydroxide or magnesium hydroxide. Typically, an alkalinity around 100 ppm is optimal for nitrifier function but there is no harm in being lower or higher assuming the system is maintaining a stable pH and nitrification is functioning well enough to handle the rate of ammonia loading in a system. 8.64 mg/L bicarbonate (HCO3) is considered adequate to remove 1 ppm of ammonia in wastewater systems based on a model of nitrification from 1976 (USEPA, 2002).
What is the process of removing nitrogen?
Definition. The removal of nitrogen by biological nitrification and denitrification is a two-step process. In the first step (nitrification), ammonia is converted aerobically to nitrate (NO 3− ). In the second step (denitrification), nitrates are converted to N 2 O or nitrogen gas (N 2) under anoxic conditions.
Which bacteria are responsible for nitrification?
Aerobic autotrophic bacteria are responsible for nitrification in activated sludge and biofilm processes; Two‐step process in nitrication involve two groups of bacteria; First stage, ammonia is oxidized to nitrite by one group (Nitrosomonas) and second stage, nitrite is oxidized to nitrate by another group of autotrophic bacteria (Nitrobacter) ...
What bacteria are oxidized to nitrate?
Other autotrophic bacteria for oxidation of nitrite to nitrate (prefix with Nitro‐): Nitrococcus, Nitrospira, Nitrospina, and Nitroeystis.
Is nitrification inhibited by ammonia?
Nitrification is also inhibited by un‐ionized ammonia (NH 3) or free ammonia, and un‐ionized nitrous acid (HNO 2 ); Inhibition effects are dependent on total nitrogen species concentration, temperature, and pH. Let us know in the comments what you think about the concepts in this article!
What are nitrifying bacteria?
Nitrifying bacteria are autotrophs, they use inorganic sources of carbon (such as carbon dioxide and carbonate ion) to produce biomass in contrast to the great majority of the other microbes in the treatment system (heterotrophs) which typically use a variety of organic substances both as an energy and carbon source.
How long does it take for a heterotroph to divide?
Nitrosomonas may reproduce (divide) once in eight hours compared to a fast-growing heterotroph that may divide every 20 minutes.
What is the recommended oxygen concentration for autotrophs?
A dissolved oxygen concentration of 2 mg/L should be maintained. Keeping high concentrations of substances known to be toxic to the autotrophs such as excessively high ammonia concentrations or toxic heavy metal ions such as copper and chromium out of the wastewater entering the system.
Is it possible to maintain optimal conditions in a wastewater treatment plant?
Maintaining optimal conditions in a wastewater treatment plant is not always practical since it can be cost prohibitive. Instead, several measures can be used to help maintain the nitrifying populations in the face of variable conditions.
Ammonifiable organic nitrogen
It is the most common form and ammoniacal nitrogen (NH4-H). Indeed, it is the one present in the urine.
Biological nitrification
This is the biological cycle of transformation of reduced nitrogen into the oxidized form nitrate (NO3-). And micro-organisms play a major role in this process.
Biological denitrification
The purpose of biological denitrification is to completely remove nitrogen from wastewater. During this treatment process, the nitrogen evaporates into the atmosphere in its molecular form N2.
What is biological nitrification?
Biological nitrification is the microbe-mediated process of oxidizing ammonia to remove nitrogenous compounds from wastewaters. Domestic sewage typically contains 20 to 40 mg/L of ammonia nitrogen (NH 4- N). Organic matter containing nitrogen, e.g., protein and nucleic acid, also biodegrades to release ammonia.
What are the operations of nitrifying bacteria?
Operational practices that ensure short residence time and circulation within the system can minimize nitrification problems.
What is the process of oxidizing ammonia to nitrate?
As shown in the nitrification process , ammonia is first oxidized to nitrite ions, then the nitrite ions are oxidized to nitrate ions. Each oxidation is carried out by a different group of bacteria, the ammonia oxidizing bacteria (AOB) and the nitrite oxidizing bacteria (NOB).
What is the main cause of nitrification?
Excess nitrogen in the form of ammonia in finished water can be the principal cause of nitrification since ammonia serves as the primary substrate in the nitrificaiton process. Ammonia, nitrate and nitrite can typically be found in surface water supplies as a result of natural processes.
Why is pH important in nitrification?
First, a reduction of total alkalinity may accompany nitrification because a significant amount of bicarbonate is consumed in the conversion of ammonia to nitrite. A model that was developed in 1974 indicates that 8.64 mg/L of bicarbonate (HCO 3) will be utilized for each mg/L of ammonia-nitrogen oxidized. While reduction in alkalinty does not impose a direct public health impact, reductions in alkalinity can cause reductions in buffering capacity, which can impact pH stability and corrosivity of the water toward lead and copper. Secondly, nitrifying bacteria are very sensitive to pH. Nitrosomonas has an optimal pH between approximately 7.0 and 8.0, and the optimum pH range for Nitrobacter is approximately 7.5 to 8.0. Some utilities have reported that an increase in pH (to greater than 9) can be used to reduce the occurrence of nitrification.
How does ammonia stripping work?
Ammonia stripping is the removal of nitrogen from wastewater when the nitrogen is in gaseous ammonia form . Ammonia is a volatile substance, which means that is has a tendency to leave the wastewater and enter the atmosphere. Ammonia (NH 3) and ammonium (NH 4) exist in equilibrium with each other based on the pH. Most of the ammonia-nitrogen in municipal wastewater is in the ammonium form because of its neutral pH range (between 6 and 8). Therefore, chemicals such as lime or sodium hydroxide must be added to raise the pH to the 10.5 to 11.5 range. This will effectively "convert" the ammonium in the wastewater to ammonia. The stripping effect is achieved by introducing the high pH wastewater into th etop of a tower packed with fixed media (or "packing"). Air is blown into the bottom of the tower and flows in a countercurrent fashion with the incoming wastewater. The intimate contact between wastewater droplets and fresh air encourages the ammonia to volatilize from the wastewater to the exiting air stream.
How do bacteria remove nitrogen from wastewater?
Bacteria remove nitrogen from wastewater by a two step biological processes: nitrification followed by denitrification. Technically, it is a three step process: ammonification precedes nitrification and denitrification.
How does nitrification affect water quality?
Under certain circumstances nitrification can have a beneficial impact on drinking water quality. This would be true in the controlled conditions at a water treatment plant. Rittmann and Snoeyink (1984) reported that nitrification of ammonia-containing groundwater resulted in "biologically stable" water that did not permit bacterial growth in the distribution system. Kurtz-Crooks et al. (1986) showed that the chlorine demand of ammonia-laden groundwater was reduced after nitrification. Nitrification was found to lower treatment costs and reduce formation of trihalomethanes.
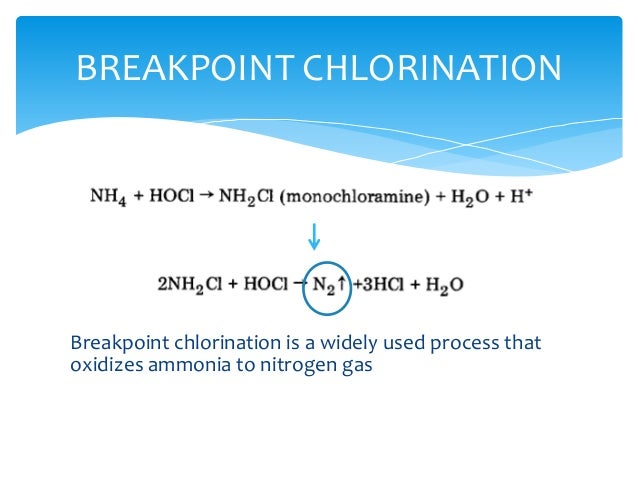
What is nitrification mitigation?
Nitrification mitigation techniques such as break-point chlorination or temporarily switching from chloramines to free chlorine can result in increased levels of disinfection by-products. Disinfection by-products are regulated under the Stage 1 Disinfectants and Disinfection By-Product Rule. Under the Disinfectants and Disinfection By-Product Rule compliance monitoring samples are be collected under routine operating conditions. Thus, disinfection by-product samples collected during a nitrification mitigation episode are not typically included in MCL compliance calculations. It would also be possible to exceed a Maximum Residual Disinfectant Level (MRDL) under the Disinfection By-Product Rule during a nitrification mitigation episode. However, the USEPA specifically allows short-term exceedence of MRDLs to control microbiological contamination problems.
What is the EPA phase 2 nitrate?
The EPA Phase II Inorganic Contaminant regulations require water systems to sample for nitrite and nitrate at each entry point to the distribution system on at least an annual basis. Additional monitoring is required on a quarterly basis for at least one year following any one routine sample in which the measured concentration is greater than 50 percent of the MCL (EPA 2001). The MCLs are 1 mg/L for nitrite-N, 10 mg/L for nitrate-N, and 10 mg/L for nitrite + nitrate (as N).
What is the main cause of nitrification?
Excess nitrogen in the form of ammonia in finished water can be the principal cause of nitrification since ammonia serves as the primary substrate in the nitrification process. Ammonia, nitrate and nitrite can typically be found in surface water supplies as a result of natural processes. These natural sources of nitrogen generally have minimal impacts on water supply distribution systems because the concentration of nitrite nitrogen in surface and ground waters is normally far below 0.1 mg/L (Sawyer and McCarty, 1978). Other sources of nitrogen can include agricultural runoff from fertilization or livestock wastes or contamination from sewage. Ammonia also occurs naturally in some groundwater supplies, and groundwater can become contaminated with nitrogen as agriculture runoff percolates into aquifers. A survey of
Does nitrification affect pH?
Nitrification can have the adverse impacts of increasing nitrite and nitrate levels, reducing alkalinity, pH, dissolved oxygen, and chloramine residuals, and promoting bacterial regrowth (Wilczak et al. 1996). Table 1 provides a summary of water quality problems associated with nitrification.
Does monochloramine degrade?
Although monochloramine will degrade when exposed to the atmosphere at varying rates depending on the amount of sunlight, wind, and temperature (Wilczak, 2001), nitrifiers are very sensitive to near UV, visual, and fluorescent light; consequently, nitrification episodes in distribution systems occur in the dark (in covered reservoirs, pipelines, taps, etc.) (Wolfe et al, 2001). Wolfe et al (2001) also report that nitrifiers do have an excision repair mechanism for DNA repair; therefore low levels of nitrifiers may be recovered from partially shaded reservoirs or channels.

Definition
Purpose of Nitrification
- Effect of ammonia on receiving water with respect to DO concentrations and fish toxicity
- Need to provide nitrogen removal to control eutrophication
- Need to provide nitrogen control for water‐reuse applications including groundwater recharge
- Drinking water maximum MCL for nitrate nitrogen is 45 mg/L as nitrate or 10 mg/L as nitrogen
Nitrification Process
- Nitrification process in waste water treatment is accomplished in both suspended growth and attached growth biological processes
Factors Affecting Process of Nitrification
- Environmental Factors: pH
1. Nitrification process in waste water treatment is pH sensitive and rates decline significantly at pH values below 6.8; Optimal nitrification rates occur at pH values in 7.5‐8.0 range; pH of 7.0 to 7.2 is normally used; 2. Low alkaline waters require alkalinity to be added to maintain acceptabl… - Environmental Factors: Toxicity
1. Nitrifiers are good indicators of presence of organic toxic compounds at low concentrations; 2. Toxic compounds include: Solvent organic chemicals, amines, proteins, tannins, phenolic compounds, alcohols, cyanates, ethers, carbamates, and benzene