
Full Answer
What is the reaction between NBS and cyclohexene?
NBS provides a low concentration of Br 2 through its reaction with HBr (eq 2). Br 2 then reacts with the substrate (RH) (cyclohexene) by a radical chain mechanism (eq 3-4) to form the brominated product (RBr) and HBr, which reacts immediately with NBS to form more Br 2 (eq 2).
How do you complete the mechanism and the product of cyclohexene?
Use curved arrows, bonds, atoms, and electrons to complete the mechanism and product (s). The first two steps are completed. Question: Complete the mechanism and the products for the reaction of cyclohexene with N-bromosuccinimide (NBS) in light and carbon tetrachloride.
How do you make 3-bromocyclohexene?
Relevant textbook readings– Klein, Chapter 11 Overview – Cyclohexene will be reacted with N-bromosuccinimide(NBS) to form 3-bromocyclohexene. Benzoyl peroxide (BPO) will be used as a radical initiator and cyclohexane will be used as a solvent.
What is the mechanism of action of NBS?
NBS provides a low concentration of Br2through its reaction with HBr(eq 2). Br2then reacts with the substrate (RH) (cyclohexene)by a radical chain mechanism (eq 3-4) to form the brominated product (RBr) and HBr, which reacts immediately with NBS to form more Br2(eq 2).
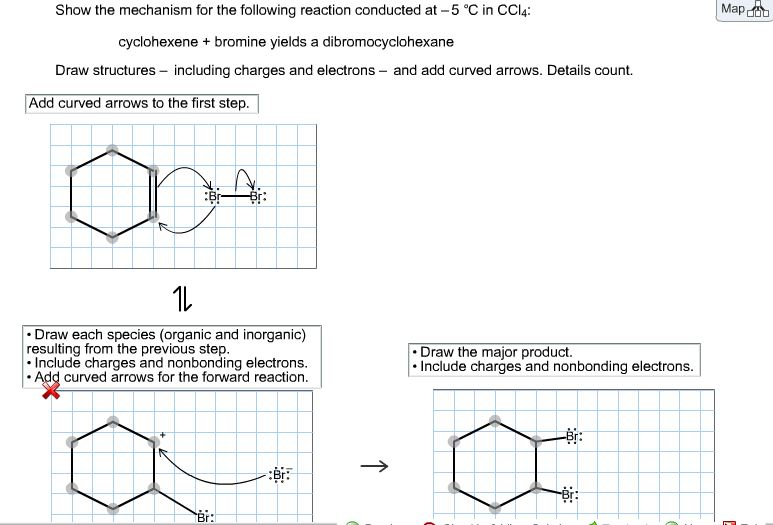
What happens when cyclohexene reacts with NBS?
Overview – Cyclohexene will be reacted with N-bromosuccinimide (NBS) to form 3-bromocyclohexene. Benzoyl peroxide (BPO) will be used as a radical initiator and cyclohexane will be used as a solvent.
What does NBS do in a reaction?
N-Bromosuccinimide (NBS) is a brominating and oxidizing agent that is used as source for bromine in radical reactions (for example: allylic brominations) and various electrophilic additions.
What does NBS do to an alkene?
NBS As A Reagent For Bromohydrin Formation From Alkenes When water (or an alcohol) is used as a solvent, it will attack the bromonium ion, resulting in formation of the halohydrin.
What is the major product when cyclohexene react with NBS A B C D Save & Next?
The IUPAC name of the product is 1-(bromomethyl)cyclohexene. So, the correct answer is “Option C”. Note: In the above reaction, we see that along with NBS, a peroxide is used usually. This is because peroxide is used as a radical initiator.
What happens when ethanol reacts with NBS give chemical reaction?
One can selectively oxidise secondary alcohols in the presence of primary alcohols using NBS in aqueous dimethoxyethane (DME). So secondary alcohol will be oxidised to a ketone.
Which solvent is most commonly used with NBS?
NBS will react with alkenes 1 in aqueous solvents to give bromohydrins 2. The preferred conditions are the portionwise addition of NBS to a solution of the alkene in 50% aqueous DMSO, DME, THF, or tert-butanol at 0 °C.
Does NBS add to the allylic position?
2:283:55Allylic Bromination Using NBS - YouTubeYouTubeStart of suggested clipEnd of suggested clipThey both will contribute to the way the structure look and the structure will be more stable thanMoreThey both will contribute to the way the structure look and the structure will be more stable than normal to explain why we have the reach of selectivity at the allylic position.
What does Lindlar's catalyst do?
Lindlar's Catalyst transforms an alkyne to a cis-alkene because the hydrogenation reaction is occurring on the surface of the metal. Both hydrogen atoms are added to the same side of the alkyne as shown in the syn-addition mechanism for hydrogenation of alkenes in the previous chapter.
How do you crystallize NBS?
In an efficient fume hood (caution: bromine evolution), an impure sample of NBS (200 g) is dissolved as quickly as possible in 2.5 L of preheated water at 90–95 °C. As filtration is usually unnecessary, the solution is then chilled well in an ice bath to effect crystallization.
Which of the following is the major product of the following reaction Ch₂ NBS?
The major product formed is 3-bromo 1-methyl cyclohexene. Minor Products formed: so option (B) is correct.
Is treated with NBS the product formed is?
NBS(N-bromosuccinimide) is used for allylic bromination. The given compound in equation undergoes restrictive allylic bromination due to the low concentration of Br2 because N-bromosuccinimide (NBS) is present with a trace amount of acid (HBr), HBr will react with NBS to give succinimide and Br2.
Which of the following is the major product formed on the reaction of cyclohexene with Br2?
1)The product of reaction betweem cyclohexene and Br2 in the presence of Uv light is 3 bromo cyclohexene.