
What is a double-blind research study?
Who is blinded in a double-blind study?
What is given to patients in a double blind trial?
A double blind trial is a trial where neither the researchers nor the patients know what they are getting. The computer gives each patient a code number. And the code numbers are then allocated to the treatment groups. Your treatment arrives with your code number on it.Feb 1, 2022
What is a double-blind study quizlet?
Who gets the placebo in an experiment?
Who should be blinded in a clinical trial?
Is Allocation to treatment groups concealed?
When do you use intention to treat analysis?
What does intention to treat mean in research?
What is a double-blind procedure in psychology quizlet?
Which individuals in a double-blind trial are unaware of who is in the control and test groups ?'?
What is meant by blinding and double blinding quizlet?
What is blinding?
Blinding means hiding who is assigned to the treatment group and who is assigned to the control group in an experiment .
What is the difference between single-blind, double-blind and triple-blind studies?
In a single-blind study , only the participants are blinded. In a double-blind study , both participants and experimenters are blinded. In a tri...
Why is blinding important?
Blinding is important to reduce bias (e.g., observer bias , demand characteristics ) and ensure a study’s internal validity . If participants kn...
Why do we do double blind studies?
Reasons to Use a Double-Blind Study 1 First, since the participants do not know which group they are in, their beliefs about the treatment are less likely to influence the outcome. 2 Second, since researchers are unaware of which subjects are receiving the real treatment, they are less likely to accidentally reveal subtle clues that might influence the outcome of the research. 1
What does double blind mean in psychology?
As mentioned previously, double-blind indicates that the participants and the experimenters are unaware of who is receiving the real treatment . 1 What exactly do we mean by ‘treatment'? In a psychology experiment, the treatment is the level of the independent variable that the experimenters are manipulating.
What is a placebo pill?
A placebo is an inert substance, such as a sugar pill, that has no effect on the individual taking it. The placebo pill is given to participants who are randomly assigned to the control group.
Why is double blinding important?
A double-blind study can be a useful research tool in psychology and other scientific areas. By keeping both the experimenters and the participants blind, bias is less likely to influence the results of the experiment.
Why is randomized double blind placebo considered the gold standard?
2 One of the reasons for this is the fact that random assignment reduces the influence of confounding variables.
Why do we use double blind?
The double-blind procedure helps minimize the possible effects of experimenter bias. 2 Such biases often involve the researchers unknowingly influencing the results during the administration or data collection stages of the experiment. Researchers sometimes have subjective feelings and biases that might have an influence on how the subjects respond or how the data is collected.
Can you double blind in a psychotherapy experiment?
Double-blind experiments are simply not possible in some scenarios. For example, in an experiment looking at which type of psychotherapy is the most effective, it would be impossible to keep participants in the dark about whether or not they actually received therapy.
What is double blinding in clinical trials?
A single-blind study masks the subjects from knowing which study treatment, if any, they are receiving. A double-blind study blinds both the subjects as well as the researchers to the treatment allocation. Triple-blinding involves withholding this information from the patients, researchers, as well as data analysts.Randomized, double-blind placebo-controlled trials involve the random placement of participants into two groups; an experimental group that receives the investigational treatment and a control group that acquires a placebo. Neither the researchers nor the study subjects know who is getting the experimental treatment and who is getting a placebo. This type of clinical study ranks as the gold standard for the validation of treatment interventions. [3]
Why do we have to double blind?
The double-blinded study minimizes the risk of various types of biases, such as observer bias or confirmation bias, which may influence the results of the investigation. [5][6] It may also help avoid a disproportionately large placebo effect in the patients involved in the study. [3]
When does unblinding occur?
Unblinding may occur during any portion of the blinded clinical trial. Unblinding that occurs before the conclusion of a trial may be a source of bias that the study should document and report. It is the responsibility of all the healthcare professionals involved in a clinical trial, such as physicians, nurses, pharmacists, technicians, and data analysts, to maintain blinding as effectively as possible during the trial and to report any premature unblinding. [7]
What is clinical research?
A clinical research study or a clinical trial is an experiment or observation performed on human subjects to generate data on the safety and efficacy of various biomedical and behavioral interventions. [1]
What is NCBI bookshelf?
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Is blinding possible in clinical trials?
Unfortunately, blinding is not possible to achieve in all clinical trials. For example, the method of drug delivery may not be amenable to blinding. An excellent clinical protocol may help ensure that within the ethical and practical constraints, blinding is achieved as effectively as possible.
Is hydroxychloroquine a double blind trial?
Controlled, double-blind, randomized trial to assess the efficacy and safety of hydroxychloroquine chemoprophylaxis in SARS CoV2 infection in healthcare personnel in the hospital setting: A structured summary of a study protocol for a randomised controlled trial.
What is double blind in clinical trials?
In the context of a clinical trial, double-blind means that neither the patients nor the researchers know who is getting a placebo and who is getting the treatment. Because patients don't know what they're getting, their belief about what will happen doesn't taint the results. Because the researchers don't know either, they can't hint ...
What is clinical trial?
A clinical trial is one that involves human participants and seeks to answer specific questions about a type of medical intervention. This can be a drug or other type of treatment, such as nutritional changes or massage. 1 . Jose Luis Pelaez Inc / Getty Images.
What is a placebo controlled group?
Placebo-controlled refers to a control group receiving a placebo. This sets it apart from studies that simply give participants treatment and record the results.
What does it mean when a doctor is not aware of the results of a double blind study?
Similarly, the doctors not being aware of the treatments means they do not unconsciously bias their interpretation of the study results.
Why do we need double blind trials?
Double-blind trials are usually needed for drugs and treatments to get approval to be used in many countries. However, good, comprehensive double-blind trials take time and require many participants.
Why is it important to keep patients blind during the trial?
Another topic of discussion that has come about as a result of COVID-19 is the ethics of keeping patients blind during the trial as vaccine effectivity is supported. Whilst keeping the blind aspect is essential to achieving valuable and reliable information about long-term effects, there is an argument that blind participants who have received a placebo should be able to receive a vaccine as more become available.
Why are double blind trials considered reliable?
Double-blind trials are seen as the most reliable type of study because they involve neither the participant nor the doctor knowing who has received what treatment. The aim of this is to minimize the placebo effect and minimize bias .
Why do double blind trials work?
The main principle behind double-blind and randomized trials, as opposed to simple blind trials, is to avoid bias in the treatment or experimental set-up. For example, if researchers are aware of the different treatment groups are getting, they may avoid assigning more unwell patients to the treatment group. Therefore, any effect seen by the treatment may have been related to how unwell a patient was to start with, rather than the efficacy of the drug.
How many people are in a double blind trial?
To be effective, it is generally recommended that double-blind trials include around 100-300 people. If treatments are highly effective, ...
What is double blind treatment?
In double-blind trials, the treatment patients have is unknown to both patients and doctors until after the study is concluded. This differs from other types of trials, such as simple blind trials where only the patients are unaware of the treatment they are receiving, whereas the doctors know.
What is single blind experiment?
In single-blind experiments, subjects do not know which treatment they are receiving. A single-blind experiment is an experiment in which subjects are not told which of the treatment conditions they are in ; a procedure used to control demand characteristics.
What is unaware of the treatments given to subjects?
experimenters are unaware of the treatments given to subjects.
Why do experimenters want subjects to be naive?
Experimenters generally want subjects to be as naive as possible concerning the experimental hypothesis to reduce confounding by demand characteristics. Demand characteristics are the aspects of the experimental situation itself that demand or elicit particular behaviors; can lead to distorted data by compelling subjects to produce responses that conform to what subjects believe is expected of them in the experiment.
Why did James run half the subjects in each condition?
Although James wanted to run all the subjects in his experiment by himself, he realized that he couldn't complete the experiment in time without a second experimenter. To control for experimenter personality , he ran half the subjects in each condition and his roommate ran the other half. This strategy is called
How did Tim prevent noise from distracting his subjects?
Tim prevented noise from distracting his subjects by testing them in a soundproof room. This illustrates the control technique called elimination. Elimination is a technique to control extraneous variables by removing them
What encourages subjects to guess the experimental hypothesis?
experimenters encourage subjects to guess the experimental hypothesis.
Why did Kenny run all treatment conditions during the evening?
Kenny ran all treatment conditions during the evening to control for the effect of time of day. He used the technique of constancy of conditions. Constancy of conditions is a control procedure used to avoid confounding; keeping all aspects of the treatment conditions identical except for the independent variable that is being manipulated.
Who randomly assigned his abnormal psychology students to participate in a study of memory encoding specificity?
Dr. Tuten randomly assigned his abnormal psychology students to participate in a study of memory encoding specificity. This means that:
What is it called when you explain the purpose of the research and answering participants' questions at the end of the study?
Explaining the purpose of the research and answering participants' questions at the end of the study is known as: debriefing.
Why is longitudinal study better than cross sectional study?
One of the advantages of conducting a longitudinal study instead of a cross-sectional study is that: it allows researchers to follow groups long enough to assess both short-term and long-term effects.
What is single case experimental design?
single-case experimental design study participants receive some type of intervention or manipulation, and behaviors are measured more than once.
Why do psychologists notice that one client tends to arrive late for appointments?
A psychologist notices that one client tends to arrive late for appointments if the client discussed emotionally difficult material during the previous session. The term for this research method is
Do case studies allow us to draw conclusions about cause and effect?
c. Case studies do not allow us to draw conclusions about cause and effect.
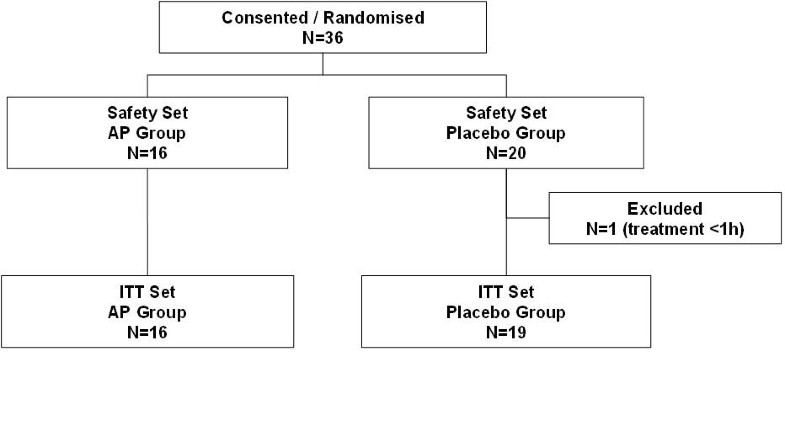
How They Work
Benefits of Double-Blind Trials
- Double-blind trials remove any power of suggestion, as no one involved knows the treatment patients receive. This means that doctors carrying out the study do not know and cannot accidentally tip off participants. Similarly, the doctors not being aware of the treatments means they do not unconsciously bias their interpretation of the study results. The main principle behin…
Covid-19 and Double-Blind Trials
- Double-blind trials are usually needed for drugs and treatments to get approval to be used in many countries. However, good, comprehensive double-blind trials take time and require many participants. This has been especially problematic during the COVID-19 pandemic, as the world has searched for pharmaceutical treatment options to improve survival and for vaccines to prev…
References
- Cancer Research UK. 2019. Randomized Trials. [online] Available at: <https://www.cancerresearchuk.org/find-a-clinical-trial/what-clinical-trials-are/randomised-trials> [Accessed 25 July 2020].
- European Centre for Disease Prevention and Control. 2020. Vaccines And Treatment Of COVID-19. [online] Available at: <https://www.ecdc.europa.eu/en/covid-19/latest-evidence/va…
- Cancer Research UK. 2019. Randomized Trials. [online] Available at: <https://www.cancerresearchuk.org/find-a-clinical-trial/what-clinical-trials-are/randomised-trials> [Accessed 25 July 2020].
- European Centre for Disease Prevention and Control. 2020. Vaccines And Treatment Of COVID-19. [online] Available at: <https://www.ecdc.europa.eu/en/covid-19/latest-evidence/vaccines-and-treatment>...
- Misra, S., 2012. Randomized double-blind placebo control studies, the "Gold Standard" in intervention-based studies. Indian Journal of Sexually Transmitted Diseases and AIDS, 33(2), pp. 131.
- The New York Times. 2021. Coronavirus Drug and Treatment Tracker [online] Available at htt…