How soon should you get monoclonal antibodies?
“It is indicated for people who are high-risk, so not everybody diagnosed with COVID qualifies for monoclonal antibody treatment,” said Dr. Turner Overton at UAB. They have to be given within a couple days of diagnosis. “And it’s really I think been life-saving and helped reduce hospitalization,” said Overton.
How often can you get monoclonal antibodies?
mAb treatment must be given within 10 days of a person’s first symptoms of COVID-19 or having been exposed to someone who has tested positive for COVID-19. The sooner a person receives mAb treatment, the better. Are mAb treatments safe? mAb treatments are still being studied.
When to get monoclonal antibody infusion?
Monoclonal antibody infusion is a COVID-19 treatment for people considered ... “We have probably 1,500 people in line waiting to get treatment,” said Shay-Dagenais. The limited number of allocations given to public health departments has added to ...
When should monoclonal antibodies be given?
Monoclonal antibodies, or mAbs, are made in a laboratory to fight a particular infection (in this case, SARS-CoV-2) and are given to you directly in an infusion. So the mAb treatment may help if you are at high risk for serious symptoms or a hospital stay.

What is monoclonal antibody?
A monoclonal antibody is a laboratory-produced protein that functions like the antibodies made by the immune system in response to infection.
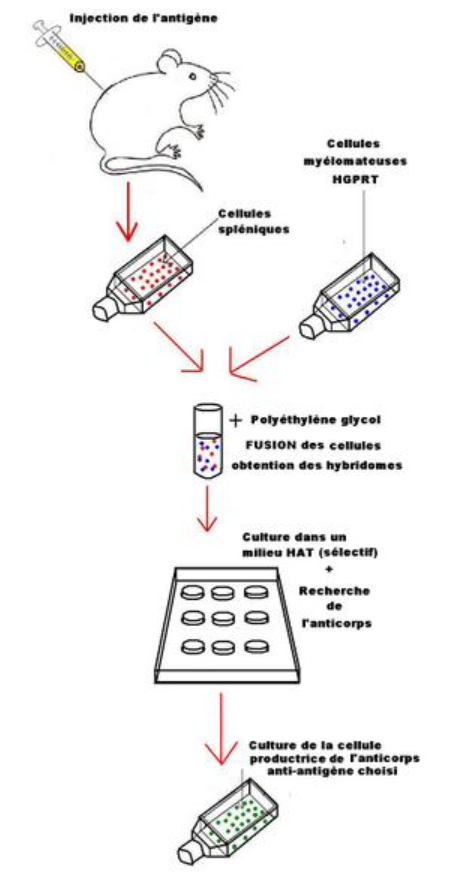
How do scientists develop monoclonal antibodies?
Scientists sometimes develop monoclonal antibodies by isolating certain immune cells — called B cells — from a person who has successfully recovered from an infection.
What antibody can help prevent the virus from infecting human cells?
By binding to the spike protein, a monoclonal antibody can help prevent the virus from infecting human cells.
Why do scientists use isolated B cells?
Scientists use isolated B cells to recreate monoclonal antibodies in a laboratory. This can be mass produced and given to people through an IV.
What is Actemra approved for?
The FDA has also given emergency authorization to Actemra (tocilizumab) for the treatment of COVID-19 in hospitalized adults and children 2 years and older. This monoclonal antibody reduces inflammation that occurs during COVID-19.
Why did the government stop distributing Bamlanivimab?
government paused distribution of these two monoclonal antibodies because tests showed that they did not work against the Beta and Gamma variants of the coronavirus. The FDA recommends that health professionals use other monoclonal antibodies instead.
How much does Regeneron cost?
The cost of Regeneron’s two-drug cocktail is $1,250 per infusion, according to Kaiser Health News. The federal government currently covers this.
How is monoclonal antibody or antiviral treatment done?
At this time, UCHealth uses Sotrovimab, which is available by FDA Emergency Use Authorization.
Where are monoclonal antibodies made?
Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
What is the function of antibodies?
Antibodies are proteins that exist in our bodies as part of our immune system to recognize and defend against harmful viruses and bacteria. Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
Can you get monoclonal antibody treatment at UCHealth?
Please speak with your health care provider or schedule a visit with UCHealth Virtual Urgent Care to determine if you are eligible for monoclonal antibody treatment and to discuss if it may be right for you. Patients who are at high risk and most likely to benefit from this treatment may be considered. Those not belonging to one of the high-risk groups will not be considered under the FDA guidance at this time.
How often is a monoclonal antibody infusion good?
While COVID-19 vaccines give you lasting protection, a monoclonal antibody infusion “is really maybe good only once or twice, ” Fuller said. “You cannot rely on it repeatedly to protect you from COVID.”
What is monoclonal antibody?
Monoclonal antibody treatments are infusions of lab-made proteins that mimic the immune system’s ability to fight off COVID. Although the Food and Drug Administration gave these treatments — like Regeneron — emergency use authorization in 2020, the criteria for who is eligible to receive them has expanded.
What happens when monoclonal antibodies enter the body?
After entering your body, monoclonal antibodies find and bind to the spike protein of the SARS-CoV-2 virus , which causes COVID-19. Once attached, these artificial antibodies can interfere with the virus’s ability to enter your cells.
Is monoclonal antibody treatment available?
Monoclonal antibody treatment is available to millions of Americans, but many may not know that. (Photo: SOPA Images via Getty Images)
Who maintains the national database of treatments?
The Department of Health and Human Services maintains a national database of where you can access to the treatments.
Is monoclonal antibody free?
The federal government is covering the cost of the monoclonal antibody therapies, so it is free to get, but there might be an administration cost billed to your insurance if you have one.
Is the infusion effective?
University of Alabama at Birmingham Professor Turner Overton, M.D., says the infusion is effective; but people still need to be vaccinated to receive the strongest protection against hospitalization due to COVID-19.
Is monoclonal antibody infusion effective?
Monoclonal antibody infusion is effective, but UAB doctors say getting the COVID-19 vaccine is the best way to prevent someone from being hospitalized because of COVID-19.
What is the purpose of monoclonal antibodies?
Monoclonal antibodies targeting the S protein have the potential to prevent SARS-CoV-2 infection and to alleviate symptoms and limit progression to severe disease in patients with mild to moderate COVID-19, particularly in those who have not yet developed an endogenous antibody response. 3.
How long should you monitor after IV infusion?
Patients should be monitored during the IV infusion or SQ injections and for at least 1 hour after the infusion or injections are completed.
What antibody targets the RBD of the S protein?
Bamlanivimab (also known as LY-CoV555 and LY3819253) is a neutralizing monoclonal antibody that targets the RBD of the S protein of SARS-CoV-2. Etesevimab (also known as LY-CoV016 and LY3832479) is another neutralizing monoclonal antibody that binds to a different but overlapping epitope in the RBD of the SARS-CoV-2 S protein. Casirivimab (previously REGN10933) and imdevimab (previously REGN10987) are recombinant human monoclonal antibodies that bind to nonoverlapping epitopes of the S protein RBD of SARS-CoV-2.
Does anti-SARS reduce the risk of death?
In randomized, placebo-controlled trials in nonhospitalized patients who had mild to moderate COVID-19 symptoms and certain risk factors for disease progression, the use of anti-SARS-CoV-2 mAb products reduced the risk of hospitalization and death (see Table 3a ). 5-7 It is worth noting that these studies were conducted before the widespread circulation of variants of concern (VOC). The potential impact of these variants and their susceptibility to different anti-SARS-CoV-2 mAbs is discussed below.
How much Sotrovimab is given?
Sotrovimab 500 mg administered as an IV infusion.
Why was bamlanivimab plus etesevimab paused?
The distribution of bamlanivimab plus etesevimab was paused in the United States because both the Gamma (P.1) and Beta (B.1.351) variants have reduced susceptibility to bamlanivimab and etesevimab. 4 However, distribution of the agents has been reinstated in states with low rates of these and other variants that have reduced susceptibility to bamlanivimab and etesevimab. Please refer to the FDA webpage Bamlanivimab and Etesevimab Authorized States, Territories, and U.S. Jurisdictions for the latest information on bamlanivimab plus etesevimab distribution.
When will the EUA update for Casirivimab be released?
On June 3, 2021, the FDA updated the EUA for casirivimab plus imdevimab to reduce the authorized dosage for a single IV infusion from casirivimab 1,200 mg plus imdevimab 1,200 mg to casirivimab 600 mg plus imdevimab 600 mg. 6 The update also authorized SQ injection of these lower doses of casirivimab and imdevimab if an IV infusion is not feasible or would delay treatment. SQ administration requires four injections (2.5 mL per injection) at four different sites (see the FDA EUA for details).
Who should get monoclonal antibody therapy?
Monoclonal antibody treatment is now available for three specific uses:
What are the side effects of monoclonal antibody therapy?
People who receive monoclonal antibody treatment may experience pain at the injection or infusion site, including:
How are monoclonal antibodies produced?
Antibodies are naturally produced by your body to fight off infections. When your body is introduced to a new virus such as COVID-19, it does not have the antibodies to fight it off. That is where monoclonal antibodies come in. Monoclonal antibodies are created in a laboratory. They can target a particular virus or infection such as COVID-19.
What is the purpose of monoclonal antibodies?
Monoclonal antibodies are given by IV to people diagnosed with COVID-19. This therapy uses COVID-19 antibodies to help a person’s body fight off the infection. Research suggests these antibodies lower the amount of virus — the “viral load” — in a person’s body. People with lower viral loads have more mild symptoms. Reducing the viral load may help prevent hospitalization and death.
How long after infusion do you have to wait to get a shot?
After undergoing infusion therapy, you must wait 90 days before getting a COVID-19 vaccine.
How old do you have to be to take Remdesivir?
Remdesivir is an antiviral drug. It can be used in people over the age of 12 weighing 88 pounds or more. It can help speed up the recovery time for people with COVID-19.
Is therapeutics covered by a CHIP?
CHIP Coverage: Therapeutics will generally be covered under an existing benefit (drugs and biologicals or other therapeutic benefits as determined under the State Plan).
How long after a positive test can you quarantine?
Stay home for your quarantine time period, which is typically 10 days after your positive test.
How do I receive treatment?
It's given by intravenous infusion, or IV. (An IV is a needle with a small plastic tube that's placed into your vein.)
Can pregnant or breastfeeding patients get monoclonal antibody therapy?
We recommend you talk about the risks and benefits with your doctor.
What is monoclonal antibody infusion?
The center has locations in Barron and Eau Claire. "A monoclonal antibody infusion is meant to boost your own body's immune system. These man-made antibodies are meant to mimic antibodies your immune system begins to make after being exposed to COVID-19," says Lori Arndt, a physician assistant in Infectious Diseases at Mayo Clinic Health System in ...
Why did the Wachsmuths get monoclonal antibodies?
The Wachsmuths qualified for the monoclonal antibodies due to age and other chronic health conditions that increased their chances of developing severe disease or requiring hospitalization. The day after their positive COVID-19 tests, Bob and Joyce received monoclonal antibody infusions at the same time in the same room at the clinic.
How long does it take for Bob to feel better after a blood test?
After the antibody infusion, Bob's symptoms continued to improve. Within several hours, Joyce began to feel much better, with no fever, chills or body aches. Lori says that their experience is consistent with other patients. "Most patients report improvement of symptoms with 24 to 48 hours after infusion," she says.
When will the Mayo Clinic open?
The Mayo Clinic COVID-19 Infusion Center opened in November 2020. The center has locations in Barron and Eau Claire.
Does Mayo Clinic have masks?
For the safety of our patients, staff and visitors, Mayo Clinic has strict masking policies in place. Anyone shown without a mask was either recorded prior to COVID-19 or recorded in a non-patient care area where social distancing and other safety protocols were followed.
