How soon should you get monoclonal antibodies?
Jan 06, 2022 · Individuals qualify for monoclonal antibody treatment if: they have tested positive for COVID-19, and it has been 10 days or less since symptoms first started, and they have other health conditions that put them at higher risk.
How often can you get monoclonal antibodies?
Dec 22, 2021 · How Long Do Monoclonal Antibodies Last? The mAb treatments have been shown to “reduce the risk of COVID-19 by 81.6% several months after a single dose,” according to the UNC School of Medicine . With recent advancements in healthcare regarding these treatments, it seems mAbs may pave the way to creating a treatment for lasting immunity to COVID.
When to get monoclonal antibody infusion?
body’s natural immune response, but this can take weeks to develop enough antibodies against a virus. So, if you have the virus, the mAb treatment gives your body the antibodies it needs to protect itself. The mAb treatment does not replace the need for t …
When should monoclonal antibodies be given?
How long does the treatment take? You should plan on about two hours for your treatment. We will meet you at your car and walk you inside, collect your vitals, review your health history and prepare the medicine. The infusion itself takes around 20 minutes. After the infusion, we'll watch you for up to an hour.
Who could benefit from monoclonal antibody therapy to prevent COVID-19?
See full answerVaccines are the best way to protect against COVID-19. But some people with weakened immune systems do not produce enough antibodies after vaccination, and others are severely allergic to the vaccine. The FDA recently authorized Evusheld, a pre-exposure prophylaxis (PrEP) monoclonal antibody therapy developed by AstraZeneca, which should help prevent COVID-19 in these populations.To be eligible for Evusheld, individuals must be 12 years or older and have a moderately to severely weakened immune system, or have a history of severe adverse reactions to the COVID-19 vaccine or its components. In addition, the therapy cannot be given to someone with a current SARS-CoV-2 infection, or who has been recently exposed to someone who is infected. Evusheld is given as two consecutive shots, and evidence suggests it can help prevent symptomatic infection for at least six months.Apr 1, 2022
What is a monoclonal antibody for COVID-19?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells. Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
How long do COVID-19 antibodies last?
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.Jan 31, 2022
Is there a monoclonal antibody therapy for post COVID-19 exposure?
FDA authorizes bamlanivimab and etesevimab monoclonal antibody therapy for post-exposure prophylaxis (prevention) for COVID-19 | FDA.Sep 16, 2021
What is a monoclonal antibody?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
Can you get COVID-19 if you already had it and have antibodies?
It is important to remember that some people with antibodies to SARS-CoV-2 may become infected after vaccination (vaccine breakthrough infection) or after recovering from a past infection (reinfected).Nov 10, 2021
How long do antibodies last in people who have mild COVID-19 cases?
A UCLA study shows that in people with mild cases of COVID-19, antibodies against SARS-CoV-2 — the virus that causes the disease — drop sharply over the first three months after infection, decreasing by roughly half every 36 days. If sustained at that rate, the antibodies would disappear within about a year.
Do people produce COVID-19 antibodies after infection?
Most people who've recovered from COVID-19 do make antibodies against the virus.Jan 21, 2022
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
How many types of monoclonal antibody COVID-19 treatments are there in the US?
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
Should I get the COVID-19 vaccine if I had COVID-19?
Yes, you should be vaccinated regardless of whether you already had COVID-19.
What is the function of antibodies?
Antibodies are proteins that exist in our bodies as part of our immune system to recognize and defend against harmful viruses and bacteria. Monoclonal antibodies are made in a laboratory and designed to target a specific virus or bacteria.
Does infusion cause nausea?
Some people may experience infusion-related side effects, such as nausea and dizziness, that are short-lived and go away on their own. As with any medication, there is the potential for mild or more severe allergic reactions, which are uncommon.
NOTE: Monoclonal antibody therapy doses containing the combination of casirivimab and imdevimab are free of charge
The U.S. government signed an agreement with Regeneron, the maker of casirivimab and imdevimab, so patients that need it would not be charged. Some patients, depending on their insurance coverage, may have to pay a fee to their healthcare provider for administering the dose.
What COVID-19 treatment is there for people outside the hospital?
If you are diagnosed with COVID-19 but aren’t sick enough to be hospitalized, you may think there isn’t much you can do. It is important to:
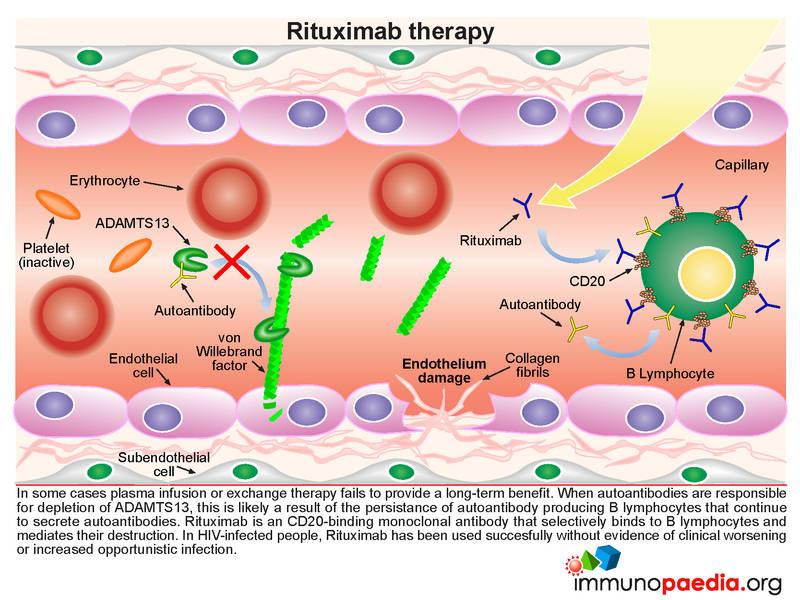
What are monoclonal antibodies?
Antibodies are naturally produced by your body to fight off infections. When your body is introduced to a new virus such as COVID-19, it does not have the antibodies to fight it off. That is where monoclonal antibodies come in. Monoclonal antibodies are created in a laboratory. They can target a particular virus or infection such as COVID-19.
How does monoclonal antibody infusion therapy work?
Monoclonal antibodies are given by IV to people diagnosed with COVID-19. This therapy uses COVID-19 antibodies to help a person’s body fight off the infection. Research suggests these antibodies lower the amount of virus — the “viral load” — in a person’s body. People with lower viral loads have more mild symptoms.
Who should get antibody infusion therapy?
Monoclonal antibodies are used for people with a positive COVID-19 test and symptoms for 10 days or less. The therapy for COVID-19 works best when given early in the COVID-19 illness. This is only recommended for those considered high risk for severe illness.
Who is at high risk for severe illness from COVID-19?
While anybody can get very sick or even die from COVID-19, those most at risk include:
What monoclonal antibody infusion therapies for COVID-19 are available?
The Food and Drug Administration (FDA) has approved emergency use authorization for four antibody infusion therapies:
