Isotonic solutions are given for:
- Hemorrhage
- Diarrhea
- Vomiting
- Fistulas (Dialysis)
- Wounds
- GI suction
- Shock
- Metabolic acidosis
- Fluid used with administering blood products
Full Answer
What is isotonic solution used for?
Isotonic solutions are used: to increase the EXTRACELLULAR fluid volume due to blood loss, surgery, dehydration, fluid loss that has been loss extracellularly. The cell has a low amount of solute extracellularly and it wants to shift inside the cell to get everything back to normal via osmosis.
What are the benefits of isotonic saline?
By maintaining consistency of cellular size, isotonic saline solutions reduce the risk of potentially-fatal health problems, like heart failure, associated with extreme rehydration. When a physical activity is considered isotonic, it involves a lifting phase and a lowering phase. Isotonic muscles, on the other hand, have the same muscle tone.
What are isotonic exercises in physical fitness?
Isotonic Exercises. When a physical activity is considered isotonic, it involves a lifting phase and a lowering phase. Isotonic muscles, on the other hand, have the same muscle tone. Bicep curls are an isotonic activity, because they involve raising the arm and lowering it, to build muscle.
What is the best isotonic solution for nursing school?
Besides the commonly known Normal Saline (aka 0.9% Saline), there are plenty of other Isotonic Solutions that you should be familiar with for nursing school. They include: 0.9% Saline (aka Normal Saline) The most obvious one is 0.9% Saline Solution, better known as Normal Saline.

What is isotonic solution used for?
Isotonic solutions are used for patients with fluid volume deficit (also called hypovolemia) to raise their blood pressure. However, infusion of too much isotonic fluid can cause excessive fluid volume (also referred to as hypervolemia).
When would you use isotonic or hypotonic?
We give them an isotonic solution to try to expand the volume of their blood but we don't want it to necessarily move solvent out of the vein into their tissues. Conversely the hypotonic solution is used when we need to put fluids into the cells for example if your patient is in Diabetic Ketoacidosis and HERE.
Why is an isotonic solution used to treat dehydration?
Isotonic IV solutions restore fluid volume because they fill the tissues and maintain fluid volume more effectively than hypertonic or hypotonic solutions.
What are isotonic IV fluids used for?
Isotonic IV Fluids Isotonic IV fluids are used to increase fluid volume due to blood loss, surgery, or dehydration . There are many different types of common isotonic fluids, such as: 1. Normal Saline (0.9% NaCl, NS) 2.
Why would you give a patient a hypertonic solution?
Clinicians use hypertonic fluids to increase intravascular fluid volume. Hypertonic saline can be utilized in the treatment of hyponatremia. Hypertonic saline and mannitol are both indicated to reduce intracranial pressure.
Which IV fluid is best for weakness?
Lactated Ringer's Solution (also known as Ringer's Lactate or Hartmann solution) is a crystalloid isotonic IV fluid designed to be the near-physiological solution of balanced electrolytes.
What fluid should be given for dehydration?
Give oral rehydration solution (ORS) immediately to dehydrated patients who can sit up and drink. If ORS is not available, you should provide water, broth, and/or other fluids. You should not provide drinks with a high sugar content, such as juice, soft drinks, or sports drinks, because they could worsen diarrhea.
When to use hypotonic solution?
Hypotonic solutions are used when the cell is dehydrated and fluids need to be put back intracellularly. This happens when patients develop diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemia.
When talking about isotonic and hypo/hypertonic, what are we talking about?
Remember when we are talking about isotonic and hypo/hypertonic we are talking about how it looks outside of the cell compared to inside.
Why do cells use osmosis?
The cell loves to be in an isotonic state and when something happens to make it unequal (like with hypotonic or hypertonic conditions) it will use osmosis to try to equal it out. Osmosis allows molecules of the solvent to pass through a semipermeable membrane from a less concentrated solution to a higher concentrated solution.
How does tonicity work in osmosis?
First, let’s get familiar with the cell and how tonicity works through osmosis. The cell is divided into two parts: ( intracellular & extracellular ). Each part is made up of a solution and depending on the tonicity of the fluid you can having shifting of fluids from outside of the cell to the inside via osmosis.
What is the meaning of tonic?
Tonic: concentration of a solution. The cell has the same concentration on the inside and outside which in normal conditions the cell’s intracellular and extracellular are both isotonic. It is important to be familiar with what fluids are isotonic and when they are given.
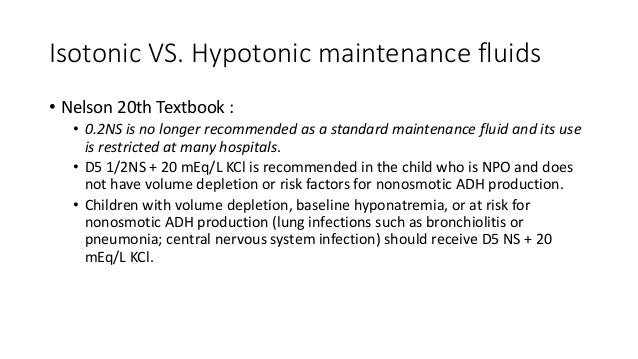
Is 5% dextrose hypotonic or isotonic?
5% dextrose in water (D5W)**also used as a hypotonic solution after it is administered because the body absorbs the dextrose BUT it is considered isotonic) Isoto nic solutions are used: to increase the EXTRACELLULAR fluid volume due to blood loss, surgery, dehydration, fluid loss that has been loss extracellularly.
Why is isotonic IV solution better than hypertonic IV solution?
Isotonic IV solutions restore fluid volume because they fill the tissues and maintain fluid volume more effectively than hypertonic or hypotonic solutions.
What is NS treatment?
Medical conditions treated with NS include fluid replacement for patients with diabetic ketoacidosis (DKA), treatment for too little sodium in the blood (hyponatremia), blood transfusions, metabolic alkalosis, and treatment for too much calcium in the blood (hypercalcemia). It is important to note that because NS replaces extracellular fluid, ...
Why is IV solution used?
IV solutions are another method to treat patients and are used to replace and control fluid and electrolyte levels in the body. These solutions are also called volume expanders. Patients suffer the loss of body fluid volume from excessive external or internal bleeding (hemorrhaging), severe burns, surgery, and dehydration, among other causes. Isotonic IV solutions restore fluid volume because they fill the tissues and maintain fluid volume more effectively than hypertonic or hypotonic solutions.
What is an IV solution?
IV solutions consist of water and various amounts of dissolved ions such as sodium (Na + ), chloride (Cl – ), potassium (K + ), magnesium (Mg 2+ ), calcium (Ca 2+ ), buffers, and other components. The solution the patient receives depends on their medical condition. The solutions shown below are all isotonic but vary slightly depending on the patient needs and appropriate uses.
What is IV therapy?
One method healthcare providers use to treat patients is via Intravenous (IV) therapy. Food, blood products, and medications are administered through a line (catheter) inserted into peripheral veins in the arms, hands, feet, or legs, or a large central vein. Peripherally inserted central catheters ...
Is red blood cell isotonic or isotonic?
The image above shows a human red blood cell in an isotonic solution. When the extracellular solution has the same solute concentration as inside the cell, the solution is isotonic. The solute concentration of isotonic IV solutions is similar to the intracellular environment.
What is isotonic solution?
The definition of “isotonic” for the purposes of nursing school is any solution that has approximately the same ratio of solute to solvent that you would measure in blood. ( Want a quick refresher on the difference between solute, solvent, and solutions?)
What is the goal of isotonic dextrose?
The Isotonic Dextrose solutions have the same goal as the Hypertonic Dextrose solutions: to provide extra calories to the patient.
What is the most commonly used IV fluid?
Isotonic solution is probably the most commonly used IV fluid that you will see during nursing school. But why is it so useful? What’s the difference between different types of isotonic fluids?
Is 5% dextrose isotonic?
5% Dextrose in Water (technically Isotonic, but it acts hypotonic …I ’ll explain in a minute)
Does isotonic solution rehydrate eggs?
It only took a few short hours to discover that the Isotonic Solution easily rehydrated my shriveled, dehydrated egg!
Can isotonic solutions reverse a problem?
Isotonic solutions can also work quickly in the body to reverse similar problems . Once the egg was rehydrated, I couldn’t tell it apart from the other hydrated eggs in my osmosis experiment.
Is dextrose isotonic or hypotonic?
Although it is technically isotonic while the Dextrose is still in the solution, the dextrose gets used up by the body’s cells for energy once it enters the blood stream. Once the dextrose is used up, all that is the water or hypotonic saline! So this remaining solution acts upon the body as if it is hypotonic.
What is isotonic hyponatremia?
Isotonic hyponatremia signifies the laboratory finding of hyponatremia in patients with no disturbances in body fluid tonicity and almost always reflects the interference of marked hyperlipidemia or marked hyperglobulinemia with certain laboratory techniques for the measurement of the plasma sodium concentration;
What causes hypertonic hypnonatremia?
Hypertonic hypnonatremia is commonly caused by hyperglycemia and rarely by infusions of hypertonic glycine.
How much sodium should be corrected for hyponatremia?
Correction of hyponatremia or hypernatremia more rapidly than 0.5 to 1.0 mEq/L/hr can lead to ODS or cerebral edema, respectively. The maximum change in serum sodium concentration should be approximately 10 mEq/L over a 24-hour period. As a rough estimation for patients weighing 70 kg, hyponatremia usually requires 450 to 550 mL per hour of isotonic 0.9% saline, while hypernatremia usually requires 200 to 300 mL per hour of hypotonic 0.45% saline to correct the serum sodium concentration by approximately 0.5 mEq per hour. Further, for hyponatremic patients with significant neurologic symptoms such as seizures, usually 150 to 250 mL of hypertonic 3% saline should be administered in the first hour to increase the serum sodium concentration by 2 mEq.
Why is hypernatremia always associated with a hyperosmolar state?
Always associated with a hyperosmolar state and occurs because of water loss in excess of salt loss; thus, there is a free water deficit, and hypernatremia is typically delineated according to the patient's ECF volume status.
Is hyponatremia a hypotonic state?
Hyponatremia does not necessarily signify a hypotonic state. Hypertonic hyponatremia occurs when there is an accumulation in the ECF compartment of non-sodium-containing effective solutes such as very high concentrations of glucose in diabetic patients or exogenously administered mannitol or glycerol. These hypertonic hyponatremic states are characterized by a shift of water from the ICF to the ECF compartments, with ICF shrinkage rather than swelling. The accumulation of a solute such as urea, which contributes to the measured plasma osmolality but is not an osmotically effective solute in terms of transcellular water shift, should not be included in the category of hypertonic hyponatremic states. Isotonic hyponatremia signifies the laboratory finding of hyponatremia in patients with no disturbances in body fluid tonicity and almost always reflects the interference of marked hyperlipidemia or marked hyperglobulinemia with certain laboratory techniques for the measurement of the plasma sodium concentration; these situations are termed pseudohyponatremia and should not prompt diagnostic or therapeutic measures to alter water balance or body tonicity.
Is sodium free hypotonic?
Retention of a sodium-free isotonic solution (e.g., infusion of isotonic mannitol in a patient with renal insufficiency who excretes the solute slowly) causes isotonic hyponatremia. Mannitol, a solute that is unable to permeate cell membranes, is confined to the extracellular space; the fluid infused with the solute is similarly confined.304 Thus, the extracellular fluid expands, and the intracellular compartment is unaffected. Sodium-free hypotonic solutions containing impermeant solutes can be considered isotonic solutions to which water has been added; hyponatremia caused by these solutions is primarily extracellular, but plasma osmolality is slightly reduced and the intracellular compartment is slightly diluted.
Is hyponatremia isotonic or hypertonic?
In contrast to hypernatremia, hyponatremia may be hypotonic, isotonic, or hypertonic. 1. 2. Euvolemic – common with renal failure, SIADH, and adrenal insufficiency. 3. Hypervolemic – caused by fluid-retaining states such as in congestive heart failure, cirrhosis, nephrotic syndrome, or renal failure.
Why is isotonic solution good for allergies?
However, because they are balanced, they make for an excellent way to rinse away allergens and provide moisture.
What is isotonic saline solution?
“Iso” means equal, and an isotonic solution should be perfectly balanced for your body. An isotonic saline solution should restore some moisture to your sinus passages. Due to a lower salt content, isotonic solutions do not draw out excess moisture ...
Can you use hypertonic saline wash?
If you experience painful burning or stinging while using a hypertonic saline wash, you should stop using it immediately. If your sinus membranes are sensitive or badly inflamed, or if the simple sensation of touch is enough to cause you pain, you should see a doctor and likely avoid using a hypertonic saline rinse.
Why is isotonic solution used?
Isotonic solution is given to ensure that the cells remain in the extracellular compartment. Goal is to increase the intravascular volume. We want to treat low extracellular fluid so it makes sense that we’d use isotonic solution to keep cells in the extracellular compartment.
How to understand hypertonic, isotonic, and hypotonic?
To understand hypertonic, isotonic, and hypotonic, you must understand the process of osmosis. With osmosis, just remember LOW to HIGH. Osmosis is the process of molecules moving from a less concentrated solution to a higher concentrated solution by passing through a semipermeable membrane.
How to treat IV fluids as a medication?
Treat IV fluids as a medication by observing for allergy response, administering to the right patient, right dosage, right route, right order, and at the right time
Which solution has a lower concentration of solutes?
Hypotonic solutions have a lower concentration of solutes.
Does hypotonic solution lower sodium levels?
Hypotonic solutions lower serum sodium levels so it’s essential to monitor sodium levels.
Overview
Dehydration is defined as the excessive loss of water from the body. The balance between fluid intake and fluid loss from the body is greatly disproportionate in dehydration. The severity of dehydration ranges from mild to severe, and dehydration can be fatal when fluid loss exceeds more than 15% of the total body water.
Pathophysiology of Dehydration
Total body water is distributed into extracellular and intracellular compartments. The extracellular compartment contains one-third of total body water and consists of the intravascular, interstitial, and transcellular spaces.
Isotonic Dehydration
Isotonic dehydration is a condition in which both water and sodium are lost proportionally and the serum sodium concentration maintains normal serum osmolality. Serum osmolality determines the movement of fluids and electrolytes across membranes. The normal serum osmolality is 285–295 mOsm/kg.
Hypertonic Dehydration
Hypertonic dehydration occurs when water excretion from the body exceeds that of sodium excretion, resulting in increased sodium concentration in the extracellular fluid (hypernatremia). Blood osmolality is increased, causing water to shift from the intracellular to the extracellular space.
Hypotonic Dehydration
Hypotonic dehydration occurs when sodium loss is greater than water loss, resulting in a decrease in serum osmolality. This causes a shift of water from the extracellular space into the intracellular space. The cells swell and cerebral edema may occur.
Isotonic and Hypotonic Fluid Disorders: Summary
Hypovolemic shock: Severe dehydration will lead to low blood volume and hypovolemic shock. It can lead to major end-organ damage through acidosis and can cause acute kidney injury which can be fatal.
Prevention of Dehydration
Adequate hydration is recommended during all activities to prevent dehydration. Water intake is key to replacing fluid lost during exercise, in hot weather, during hospitalization, and in elderly patients with impaired thirst sensation.
When to use hypotonic solution?
Conversely the hypotonic solution is used when we need to put fluids into the cells for example if your patient is in Diabetic Ketoacidosis and HERE.
What is a hypertonic IV solution?
Hypertonic, Hypotonic, Isotonic IV solutions. You want to give your patients a solution that has the tonicity that is opposite their problem most of the time. For example, if your patient is dehydrated their blood is hypertonic. They will need a hypotonic solution to bring their tonicity back within normal ranges.
What happens when you have a hypertonic to hypotonic ratio?
The ratio of hypertonic to hypotonic will create a pressure and water will pull out of the tissues into the vein to attempt to create a better ratio of water and solute (notice the water followed the salt). The same is true of people who are dehydrated.
What is hypertonic blood work?
Hypertonic (shifts fluid out of the extracellular space and into the vein, to be filtered out in the kidneys) You patient is hypotensive, dizzy, weak, and reports abdominal pain. The blood work confirms adrenal insufficiency.
Can dehydrated people use isotonic solution?
The same is true of people who are dehydrated. We give them an isotonic solution to try to expand the volume of their blood but we don’t want it to necessarily move solvent out of the vein into their tissues.
Is D5W hypertonic or isotonic?
Should you memorize this chart? NO! I put this chart here to help you visualize why the IV solution is hypertonic, hypotonic or isotonic. The number of particles in the D5W is 50, and this is a hypotonic solution. Whereas D5 + Ringer’s is a hypertonic solution and it has 361 particles.
