What is lime used for in water treatment?
Lime precipitates the CO 2 to form calcium carbonate, which provides a protective coating on the inside of water mains. Lime is used in conjunction with alum or iron salts for coagulating suspended solids in order to remove turbidity from water. It serves to maintain the proper pH for most satisfactory coagulation conditions. In some water-treatment plants, alum sludge is …
Why is ozonation not economical for wastewater treatment?
Lime is the most widely used reagent in water treatment applications. It is supplied in two forms: slaked (or hydrated) lime: Ca (OH) 2. Warning: in both cases, these reagents will contain between 4 and 20% of solid impurities (CaCO 3, SiO 2 …). These impurities must be removed before the product is used.
What is the effect of lime on corrosive water?
Aug 23, 2019 · The ozone demand is related to the level of contamination in the water. When substances in the untreated water react with ozone, part of the ozone is used up, which may leave less ozone available to treat the targeted contaminants. Ozone treatment can produce harmful by-products in drinking water. For example, if bromide is present in the raw water, ozone reacts …
How is powdered lime metered out from dilution tanks?
The ozone is passed through a diffuser which creates ozone-saturated bubbles that are mixed with water in a purification tank. The weakly-bonded atoms in the ozone will then bind themselves to bacteria, viruses, and potentially toxic metals that have been dissolved in the water; turning them into insoluble particles that can then be removed ...
Why is lime added to water during treatment?
Why lime is added after chlorination?
Why lime is added during coagulation?
Why is lime and alum used in water treatment?
How does lime affect the pH of water?
Does lime raise pH in water?
How does lime react with water?
What is lime dosing in water treatment?
What happens if lime and alum mix?
What will happen when alum is added in water?
Does alum remove fluoride from water?
What is the purpose of aeration?
What is lime used for?
Lime is the most widely used reagent in water treatment applications. It is supplied in two forms: slaked (or hydrated) lime: Ca (OH) 2. Warning: in both cases, these reagents will contain between 4 and 20% of solid impurities (CaCO 3, SiO 2 …). These impurities must be removed before the product is used.
What is slaked lime?
Slaked lime is usually used as a lime slurry, an aqueous suspension of calcium hydroxide particles. Its concentration is usually between 50 and 100 g.L -1 for ease of use; in effect:
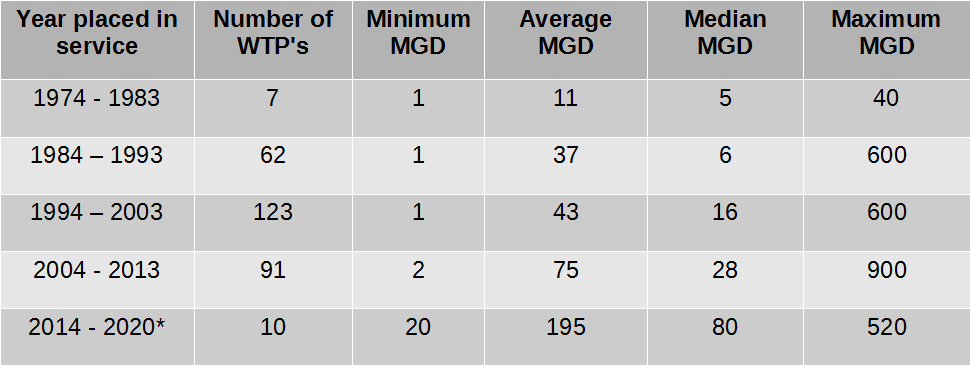
How much lime can be stored in a silo?
Powdered lime is stored in 25 kg bags or in a silo, depending on the size of the installation. In France, the preferred minimum silo capacity is 50 m³, this being the maximum lorry payload (25 tonnes).
What is propeller mixer?
A propeller-operated mixer (7) is used for recycling purposes; this mixer is placed in the top section of the nozzle into which the water to be saturated is also fed (1) and where the water + lime slurry + carbonate sludge are thoroughly mixed together.
What is ozone treatment?
Ozone may be recommended as a pretreatment to control biofouling of reverse osmosis membranes and ion exchange resin, as certain membranes and resins are degraded by chlorine. Ozone treatment units are installed as a point-of-entry treatment system. At the point where the ozone mixes with the water, turbulence and bubbles are created;
What is the effect of pH on ozone?
Ozone has been found to be effective over a wide range of pH, but a pH slightly above 7 increases treatment efficiency. The ozone demand is related to the level of contamination in the water. When substances in the untreated water react with ozone, ...
Where is treated water stored?
Some systems store treated water in a contact chamber to assure that water is continuously treated until used. Ozone treatment systems frequently have pre-treatment and post-treatment devices.
How does ozone work?
How ozone treatment works. Ozone is an unstable form of pure oxygen. Like chlorine, ozone is a strong oxidizing agent and is used in much the same way – to kill disease-causing bacteria and viruses. Ozone may not kill large cysts and some other large organisms, so these should be eliminated by filtering or other procedures prior to ozone treatment.
Is ozone better than chlorine?
It oxidizes iron and manganese into solid particles that can be filtered out. Some research has shown that ozone is more effective than chlorine or ultra-violet disinfection for treating Giardia lamblia or Cryptosporidium parvum.
Can ozone cause cancer?
Ozone treatment can produce harmful by-products in drinking water. For example, if bromide is present in the raw water, ozone reacts with it to form bromate, shown to cause cancer in rats.
Do ozone systems need maintenance?
Most ozone systems do not require extensive maintenance. Some systems use an air-drying material, which needs to be replaced periodically. It is also necessary to periodically clean the water storage tank and check pumps, fans and valves for damage and wear.
Does ozone oxidize water?
In many cases, depending on the ozone contact time and concentration, it can oxidize these contaminants to water and carbon dioxide.
Is ozone a disinfectant?
It is a powerful oxidant and one of the most powerful disinfectants available in water treatment. Drinking Water Disinfection: Although ozone is significantly more effective than chlorine at inactivating and / or killing viruses, bacteria and cysts (e.g., Cryptosporidium and Giardia) and has been widely used in Europe for many years ...
What is ozone water?
Ozonation / Ozone Water Treatment. Ozone is a form of oxygen (O2) with the molecular formula O3. It forms when oxygen in the air is exposed to the discharge of a powerful electric current through air. In nature, it forms in the upper atmosphere when lightning passes through the air. The pungent odor often associated with passage of a thunderstorm, ...
Is ozone soluble in water?
Ozone is not very soluble in water and is typically dissolved using a venture which relies on intimate air / water contact under very high turbulence or a diffuser which breaks the gas into very tiny bubbles which are allowed to be in contact with the water for an extended period of time.
Is ozone better than chlorine?
Drinking Water Disinfection: Although ozone is significantly more effective than chlorine at inactivating and / or killing viruses, bacteria and cysts (e.g., Cryptosporidium and Giardia) and has been widely used in Europe for many years to treat municipal drinking water, it has not had similar acceptance in the US.
Is ozone a reactive oxidant?
Since the free hydrox yl is so highly reactive, the contact time necessary is minimal, as compared to other disinfectants. Ozone may be combined with other oxidation processes, such as ultraviolet irradiation, hydrogen peroxide (another powerful oxidant) and proprietary catalysts to speed these oxidation process.
What is the process of oxidation called?
This combination of oxidation steps is referred to as Advanced Oxid ation Processes or AOP.
How Is Ozone Formed?
According to the Water Research Center, ozone is made up of three oxygen atoms and is formed when you apply energy (generally electricity or UV radiation) to oxygen. By passing air through an ozone generator, you can create this useful chemical, which can then be applied to water in order to remove metals, bacteria and other substances.
Why Ozone Is Recommended for Wastewater Disinfection
Said simply, ozone water treatment is a chemical-free, low-cost solution to water sterilisation or disinfection. As a resource, ozone is completely renewable and the resources involved in its production are renewable, too.
More for Your Money ? the Extra Benefits of Ozone
As well as successfully removing metals and giving new life to your wastewater, [link to post 2] ozone has a range of complementary benefits that are undoubtedly value adding. As a business, investing in these extras makes sense. As well as getting a safer, less chemically-dependant, greener worksite, you can take advantage of:
Where Is Ozone Already Used?
The applications for ozone water treatment have only been growing since the 1800s. Today, new applications are emerging all the time, with large-scale treatment options becoming a reality in areas such as:
Get the Ozone Advantage with OLEOLOGY
Based in Western Australia and servicing Australia and NZ, OLEOLOGY offers a full suite of highly effective water treatment solutions. To invest in ozone water treatment for your needs, call 1300 692 359 or contact us online.
Does photocatalytic ozonation reduce toxicity?
In addition to the degradation and mineralisation of various contaminants in water by photocatalytic ozonation, the process can also reduce contaminant toxicity in water and wastewater. The detoxification and disinfection effects of photocatalytic ozonation have been assessed by several research groups using various standard biotests. Ochiai et al. [70] described that shifting from simple ozonation to photocatalytic ozonation nearly doubled the removal rate constants for waterborne pathogens (evaluated by Escherichia coli standard kit). Beltran et al. [68] conducted acute toxicity Danphnia tests and reported decreasing the ecotoxicity of sulfamethoxazole solutions from 60% to 10% after a 120 min photocatalytic ozonation treatment. Arana et al. [71] used total coliform determination method and reported complete bacterial disinfection of natural wastewater by combining sunlight, TiO 2 and ozone, while separately, ozonation and irradiated TiO 2 did not prevent the growth of bacteria after treatment. As a measure of detoxification for 2,4-dichlorophenoxyacetic acid solutions, Giri et al. [30] reported a 3-fold greater dechlorination efficiency for photocatalytic ozonation in comparison to photocatalysis in the presence of oxygen, while the synergistic effect was more clearly observed by applying low ozone concentrations [72]. Similar effects were also reported by other research groups using Daphtoxkit microbio test and Luminescence bacteria test [46], chl- a test [73], and E. coli standard kit [74].
What is photocatalytic ozonation?
•#N#Photocatalytic ozonation is a powerful oxidation process for water/wastewater treatment .#N#•#N#Synergistic effects are observed by combining various photocatalysts with ozone.#N#•#N#Photocatalytic ozonation is often more cost-effective than ozonation and photocatalysis.#N#•#N#Photocatalytic ozonation can moderate the poor mass transfer of fixed catalysts.#N#•#N#Changing the operational conditions can significantly affect the oxidation efficiency.
What is the most commonly used photocatalyst for ozonation?
Due to its chemical stability, high photocatalytic activity and low toxicity, TiO 2 is the most reported photocatalyst used for photocatalytic ozonation processes. This photocatalyst is relatively inexpensive and commercially available as Degussa P-25, BDH, PSB-P200, TP-2, Sachtleben Hombikat UV100 and Millennium TiONA PC50 [72], [86], [87]. Some research groups reported preparation of TiO 2 [51], [62], [64] and also the modification of TiO 2 particles by doping or mixing with activated carbon [5], [71], metal oxides [3], [34], [48], [88] and noble metals [73], [89] in order to improve photocatalytic properties. However, photocatalysts other than TiO 2 are also used for photocatalytic ozonation treatments [65], [90].
How do photocatalysts affect oxidation?
The presence of photocatalysts (in addition to ozone) in the oxidation medium and the adsorption of ozone and pollutants on its surface can essentially change oxidation mechanisms which indicates photocatalytic ozonation is a different process from ozonation in the absence of a photocatalyst. Principally, photocatalytic reactions commence by photoexciting the surface of photocatalyst with UV–Vis radiation, which can provide the appropriate band gap energy to generate photoactivated electron–hole pairs (R1). In parallel, ozone molecules can adsorb on the surface of the photocatalyst via three different interactions: physical adsorption, formation of weak hydrogen bonds with surface hydroxyl groups, and molecular or dissociative adsorption into Lewis acid sites [35], each interaction resulting in the production of active oxygen radicals ( O) (R2). Huang and Li [36] showed these active oxygen radicals react with water molecules to produce hydroxyl radicals (R10) which play a key role in photocatalytic ozonation processes.
