
How is nitrogen removed from wastewater treatment plants?
Biological (activated sludge) treatment is the most widely utilised nitrogen removal technology in wastewater treatment plants (WWTPs). In biological treatment, nitrogen is removed in two steps: nitrification and de-nitrification.
What can be done to improve the nitrogen removal from WTP?
understanding of the nitrogen reactions occurring at all stages of treatment. It would also provide a baseline concentration of organic nitrogen entering the WTP to allow for removal efficiency calculations. Install temperature and dissolved oxygen sensors in the rock cells.
How do you remove nitrogen and phosphorus from water?
These remove both nitrogen and phosphorus from water. Helophyte filters can be applied in water purification of small surface waters. Ammonia can be removed from water by the so-called stripping process.
Are wastewater treatment plants nitrifying?
A municipal wastewater treatment plant with an effluent containing more than 5 mg/L TKN is not fully nitrifying. Ammonia (NH3 or NH4) – When the pH of the wastewater is acidic or neutral, the majority of the nitrogen is ammonium (NH4+); however, it is typically called ammonia, not ammonium.

How do water treatment plants remove nitrates?
Nitrates can be removed from water by reverse osmosis, distillation, or through ion exchange resin. Nitrates are difficult contaminants to eliminate from water.
How is nitrogen gas removed from water?
Until fixed nitrogen is converted back to nitrogen gas, it remains as a potential water contaminant. Anammox and denitrification are the only two processes that can remove excess fixed nitrogen by chemically changing it back to nitrogen gas.
In what form is nitrogen removed from wastewater?
Through wastewater treatment, the nitrogen is finally released back to the atmosphere as N2 gas. This nitrogen cycle is characterized by drawbacks. The energy requirement is high, and in the wastewater treatment, nitrogen is mainly converted to N2 gas and lost to the atmosphere.
How is nitrogen and phosphorus removed from wastewater?
Nitrogen and Phosphorus Removal from Wastewater Treatment Plant Effluent via Bacterial Sulfate Reduction in an Anoxic Bioreactor Packed with Wood and Iron.
What is the process of nitrogen removal?
There are two steps for removing nitrogen in biological treatment: nitrification and denitrification. In this process, nitrifiers, including ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB), convert total ammonia (free ammonia and un-ionized ammonia) to nitrate.
How is ammonia and nitrate removed from water?
Reverse osmosis (RO) can also be used to remove nitrate from water. RO is a non-selective technology in that it will remove all ionic contaminants from water, particularly those with higher valences.
How are phosphates and nitrates removed from water?
Phosphate and nitrate pollutants can be removed by chemical precipitation, biological treatment, membrane processes, electrolytic treatment, ion-exchange and adsorption process to remove these pollutants from water sources effectively.
What is the best biological process for nitrogen removal?
The most environmentally friendly is System B2 with mainstream anaerobic organics removal followed by anammox treatment.
How can nitrogen be recovered from wastewater?
Nitrogen removal takes place after primary settling through biological treatment. In addition to biological treatment for nitrogen removal, many wastewater treatment plants have implemented anaerobic digestion of municipal sludge to produce biogas and increase the dewaterability of the sludge.
What is nitrification and denitrification in wastewater treatment?
Untreated domestic wastewater contains ammonia. Nitrification is a biological process that converts ammonia to nitrite and nitrite to nitrate. If standards require that the resulting nitrate be removed, one treatment alternative is the process of denitrification, in which nitrate is reduced to nitrogen gas.
How are nutrients removed from wastewater?
Conventional wastewater nutrient removal systems transform fixed nitrogen (e.g., NO3−, TKN) to nitrogen gas (N2) through biological nitrification/denitrification, whereas phosphorus removal is accomplished through incorporation into microbial biomass and/or chemical precipitation.
Why do you monitor nitrogen in a wastewater treatment plant?
A wastewater treatment plant's principal objective is commonly the removal of nitrogen, a nutrient notorious for causing eutrophication in natural surface waters. High levels of nitrate in the environment can pose problems for both humans and animals.
What is a membrane aerated biofilm reactor?
Like activated sludge, membrane aerated biofilm reactor (MABR) wastewater treatment uses microorganisms to nitrify and denitrify wastewater, but MABR uses a self-aspirating, spirally-wound membrane with a large surface area to passively deliver oxygen instead of manually pumping air into the water. There are several key advantages to the configuration.
What is anammox in wastewater?
Anaerobic Ammonium Oxidation (Anammox) Anammox is an energy-efficient, microbiologically mediated process for nitrogen removal.
What is the process of oxidizing ammonia?
First, ammonia is oxidized to nitrite, which is then converted into nitrate in aerobic conditions. Heterotrophic or autotrophic bacteria then reduce the nitrate to benign nitrogen gas in anoxic conditions. During the process, wastewater is intensively aerated to nurture the microorganisms that break down dissolved organic matter.
How are microalgae used in wastewater?
BES uses the interaction between microbes and a solid electron acceptor to remove contaminants like nitrogen from wastewater while generating electricity.
Why is wastewater aerated?
During the process, wastewater is intensively aerated to nurture the microorganisms that break down dissolved organic matter. Some organic matter is used to grow new cells, and some is oxidized. The new cells are removed from the stream and put in settling tanks as sludge, some of which goes back to the aeration tank. The rest is waste.
What is the process of removing nitrogen from wastewater?
Here are some of the processes: Nitrification and Denitrification With Activated Sludge. The most widespread process for nitrogen removal from wastewater is the activated sludge process, which uses nitrification-den itrification to remove nitrate. First, ammonia is oxidized to nitrite, which is then converted into nitrate in aerobic conditions.
Why is activated sludge energy intensive?
Traditional activated sludge processes are highly energy intensive due to the air compression needed for aeration, and the process is demanding to maintain . Microalgae are simple cells that can double their biomass in 24 hours, producing valuable metabolites like fats, proteins, sugars, and bioactive compounds.
What is the NO3 concentration in wastewater?
As a result, effluent nitrate (NO3) concentrations of less than 3 mg /L exist in wastewaters that are fully nitrified and denitrified as well as in effluents with no nitrogen removal at all. An effluent that is fully nitrified but has not been denitrified will generally contain a nitrate (NO3).
What is TKN in wastewater?
TKN = NH4 + org-N. Ammonia (NH3 or NH4) – When the pH of the wastewater is acidic or neutral, the majority of the nitrogen is ammonium (NH4+); however, it is typically called ammonia, not ammonium. When the pH increases over 7.0, the nitrogen is mostly ammonia (NH3).
What is Sabre SBR?
The Sabre SBR provides complete nitrification of the influent ammonia during the aerated-fill and the react phases of treatment. During the beginning of a treatment cycle, wastewater is fed into the SBR with only mixing and no aeration.
How much organic nitrogen is in wastewater?
A municipal wastewater treatment plant that is effectively nitrifying generally contains less than 3 mg/L organic-Nitrogen.
What is the role of nitrogen in wastewater treatment?
Nitrogen Treatment. Controlling nitrogen discharged from wastewater treatment plants is a major factor in protecting surface waters. Nitrogen is one of the major nutrients contributing to the increased eutrophication of lakes and natural waters.
Can a Sabre SBR have nitrification?
Both nitrification and denitrification can be provided in a single-tank Sabre SBR.
Is ammonia converted to nitrate?
In the first scenario ammonia is converted to nitrate, and the nitrate is converted to nitrogen gas, and very little nitrate remains. In the second scenario, little to no ammonia is converted to nitrate, and as a result, there is very little nitrate produced.
What is the nitrogen in water?
Depending on water properties, various inorganic nitrogen compounds may be found. In aerobic waters nitrogen is mainly present as N2and NO3-, and depending on environmental conditions it may also occur as N2O, NH3, NH4+, HNO2, NO2- or HNO3. Water in coastal areas mainly contains elementary nitrogen gas (N2).
What causes algal blooms in seawater?
In seawater nitrates, nitrites and ammonia are dietary requirements for plankton, causing nitrogen concentrations to be lower at the surface than in the deep. At increasing nitrogen concentrations in surface layers, plankton production increases, leading to algal blooms. This may occur in any type of surface water.
How much nitrogen does a crop take up after fertilization?
After fertilization, crops take up a relatively small part of added nitrogen compounds, namely 25-30%. The residue ends up in groundwater and surface water through soils, because nitrates are water soluble.
Why is nitrogen important for plants?
Nitrogen is a dietary requirement for all organisms, because it is a constituent of all proteins and nucleic acids. Plants consists of approximately 7.5% nitrogen (dry mass). Nitrogen is essential for plants, and can be found in air in large amounts. This elementary nitrogen cannot be taken up directly.
What are the main sources of nitrogen in water?
The main source of nitrogen compounds in water are fertilizers that mainly contain nitrate, but also ammonia, ammonium, urea and amines. The most widely applied nitrogen fertilizers are probably NaNO3(sodium nitrate) and NH4NO3(ammonium nitrate).
What are the products of nitrogen?
Thereby other nitrogen compounds, such as nitrous oxide applied in anaesthetics, can be produced. Nitric acid, urea, hydrazine and amines are other products from nitrogen industries. Nitrogen compounds are by-products of colouring and synthetic agent production.
How to remove ammonia from water?
Helophyte filters can be applied in water purification of small surface waters. Ammonia can be removed from water by the so-called stripping process. This means removing ammonia from wastewater by means of air or steam, by gasifying it.
What is the process of converting nitrates into water?
BNR uses denitrification to convert nitrates in the wastewater into harmless water, nitrogen gas (N2) and carbon dioxide gas (CO2). Denitrification is a biological process that takes place under anoxic conditions. Denitrification occurs after aerobic bacteria have converted the ammonia to nitrate in a process called nitrification.
How does nitrogen affect aquatic life?
In surface waters, nitrogen can contribute to excessive growth of algae called algal “blooms” that can sicken or kill wildlife and harm aquatic habitats. These algal blooms, typically referred to as eutrophication, consume the dissolved oxygen in the water, causing hypoxia, leaving little or no oxygen for fish and other aquatic organisms. Algal blooms can harm aquatic plants by blocking the sunlight they need to grow. Some algal blooms produce toxins and encourage bacterial growths that can sicken people who swim in or drink the water, or who eat tainted fish or shellfish.
How does EOSI help?
Wastewater treatment plants are increasingly required by state and federal regulatory agencies to reduce the amount of nitrogen discharged into the environment, particularly in sensitive watersheds.
What is the process of converting ammonia to nitrogen?
Denitrification occurs after aerobic bacteria have converted the ammonia to nitrate in a process called nitrification. Often times there is insufficient organic material to support denitrification, therefore a supplemental carbon source, such as MicroC®, is added to the process to convert nitrate to nitrogen gas.
What is the name of the compound that reduces oxygen in the blood?
Elevated concentrations of a nitrogen compound called nitrate can cause methemoglobinemia, also known as “blue baby syndrome”, a potentially fatal illness in which the nitrates reduce the oxygen-carrying capacity of the blood.
What are the sources of nitrates and nitrites?
Other sources of nitrate and nitrite in the environment include fertilizers, atmospheric deposition, animal wastes, and sewage and septic systems .
What are the most common environmental problems?
Nitrogen. Nutrient pollution is one of our most widespread, costly and challenging environmental problems, according to the United States Environmental Protection Agency. Nutrients such as nitrogen and phosphorus are essential for the growth of all living organisms. However, excessive amounts of nutrients released to the environment by human ...
What is the process of converting NH3 to N2?
With shortcut nitrogen removal, NOB are not used to convert nitrite to nitrate; instead, a different class of bacteria, labeled anammox (anaerobic ammonia oxidation), converts NH3 into N2 in two biological steps. The first step is called nitritation, in which AOB (the same used with conventional systems) convert about half ...
Why is nitrogen removal called shortcut nitrogen removal?
The breakthrough is known as shortcut nitrogen removal because it eliminates a step in the BNR process. Instead of nitrifying ammonia and then denitrifying later, this revolutionary advancement replaces nitrification and denitrification with singlestep deammonification. Used mainly for high-strength sidestream wastewater flows, ...
What bacteria convert nitrogen to N2?
The anammox bacteria, which use nitrite as an electron acceptor, convert about 89 percent of inorganic nitrogen (ammonia and nitrite) into N2, with 11 percent left over as nitrate. Benefits Of Shortcut Nitrogen Removal.
What is the first step in wastewater treatment?
The first step is called nitritation, in which AOB (the same used with conventional systems) convert about half of the ammonia into nitrite. This partial nitritation is common in wastewater treatment. The revelation comes with step two: anaerobic deammonification.
How is NH3 oxidized?
Wastewater ammonia (NH3) is oxidized to nitrite by autotrophic ammonia-oxidizing bacteria (AOB), and the nitrite is then oxidized to nitrate by nitriteoxidizing bacteria (NOB) under aerobic conditions. Two additional steps are required to convert the nitrate into inert nitrogen gas (N2).
How much does BDP reduce carbon dioxide?
Reduces carbon dioxide emissions by 50 percent or more. Reduces sludge by at least 40 percent. Reduces physical footprint by approximately 50 percent. Reduces O&M costs by approximately 30 percent. BDP requires a carbon-to-nitrogen (C/N) ratio of just 0.17, which can be increased for higher nitrogen removal.
What is the source of nitrogen and phosphorus in wastewater?
Wastewater contains nitrogen and phosphorus from human waste, food and certain soaps and detergents. Once the water is cleaned to standards set and monitored by state and federal officials, it is typically released into a local water body, where it can become a source of nitrogen and phosphorus pollution. Some wastewater treatment plants are able ...
How to maintain a septic system?
Homeowners are responsible for maintaining their septic systems in most cases. To protect and maintain their system, homeowners should: 1 Have their system inspected regularly and pump their tank as necessary 2 Use water efficiently 3 Not dispose of household hazardous waste in sinks or toilets 4 Avoid driving vehicles or placing heavy objects on their drainfield 5 Visit EPA's decentralized wastewater (septic) systems webpage to learn more about septic systems and EPA's SepticSmart Week Program 6 Consult EPA's guide on maintaining septic systems for more information: Homeowner's Guide to Septic Systems (PDF) (9 pp, 3 MB, About PDF)
Why upgrade wastewater treatment system?
Enhanced treatment systems enable some wastewater plants to produce discharges that contain less nitrogen than plants using conventional treatment methods . Upgrading wastewater treatment systems is often expensive for municipalities and rate payers, but upgrades can pay for themselves or end up saving a plant money.
What causes a septic system to fail?
Common causes of septic system failure include aging infrastructure, inappropriate design, overloading with too much wastewater in too short a period of time and poor maintenance.
How does a septic system contribute to nutrient pollution?
Septic systems can easily become a source of nutrient pollution if not properly maintained. Most homes and businesses send their wastewater to a treatment plant where many pollutants are removed from the water. Wastewater treatment facilities in the United States process approximately 34 billion gallons of wastewater every day.
What percentage of homes in the US have septic systems?
Septic Systems. Approximately 20 percent of homes in the United States use septic systems that locally treat their wastewater. When a septic system is improperly managed, elevated nitrogen and phosphorus levels can be released into local water bodies or ground water.
Who is responsible for septic system maintenance?
Homeowners are responsible for maintaining their septic systems in most cases. To protect and maintain their system, homeowners should: Have their system inspected regularly and pump their tank as necessary. Use water efficiently. Not dispose of household hazardous waste in sinks or toilets.
Properties
Applications
Composition
Use
Formation
Diet
Mechanism
Climate
Other uses
Toxicity
Function
Safety
Prevention
Effects
Treatment
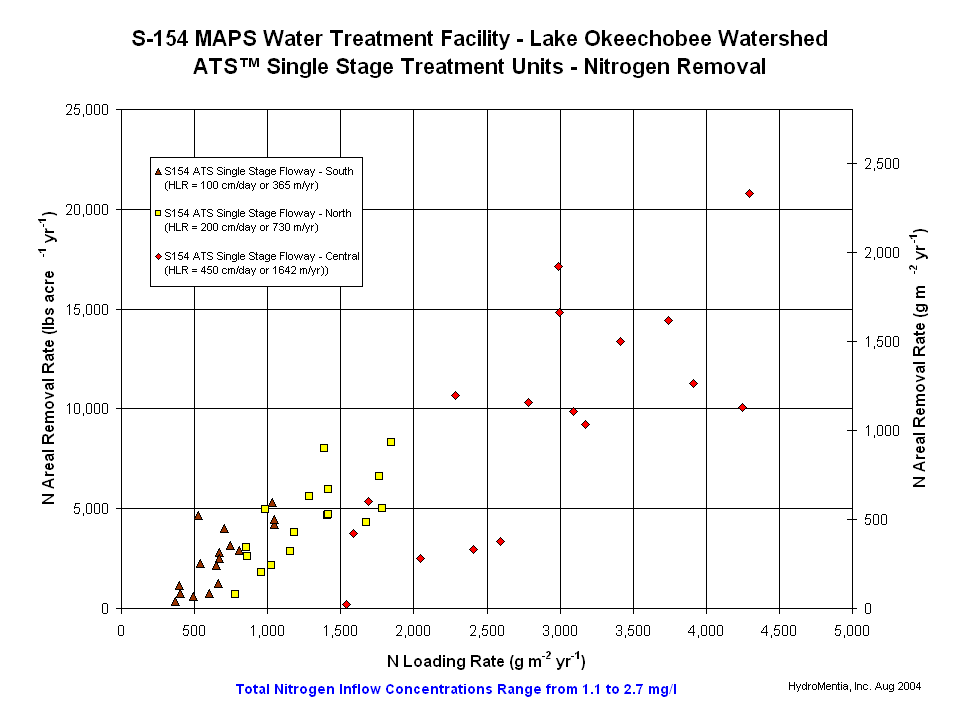
- In wastewater treatment plants the first two treatment steps may remove only 50% of nitrogen concentrations. For further treatment, lime and HOCl addition were attempted. This however turned out not to be very effective. Consequently, the third step of wastewater treatment includes biological nitrogen removal. This means a combination of nitrificat...
Synthesis
Preparation
Others