Testing the effectiveness of antimicrobial drugs against specific organisms is important in identifying their spectrum of activity and the therapeutic dosage. This type of test, generally described as antimicrobial susceptibility testing (AST), is commonly performed in a clinical laboratory.
Full Answer
How to assess antibiotic use to improve antibiotic use?
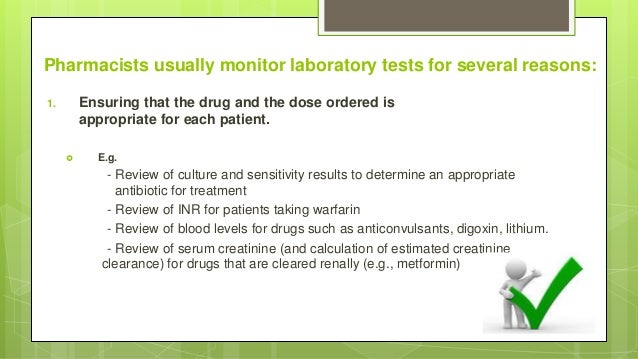
Feb 01, 2020 · Some antibiotics necessitate monitoring of drug levels to guide therapy for efficacy and prevention of adverse effects such as vancomycin and aminoglycosides. Renal toxicities may develop if these antimicrobials maintain high trough levels; therefore, monitoring renal function is necessary, in addition to measuring drug levels.
Why monitor outpatient antibiotic prescribing data?
Jun 12, 1999 · The susceptibilities of the 1234 isolates to antibiotic drugs were as follows: ampicillin 43%, cefuroxime 61%, cefotaxime 74%, gentamicin 77%, and imipenem 98%. The costs of the drug, administration, monitoring, and side effects 19 – 22 for the five drugs in our hospital are given in table. table2. 2.
What are the clinical implications of antibiotic efficacy?
Fortunately, most antimicrobial agents have a wide therapeutic index, 20 allowing standard doses to be used, with predictable modifications on the basis of age, weight, and renal and hepatic function. However, certain antimicrobial agents require monitoring of serum levels because the therapeutic window is narrow.
What determines the level of aggressiveness in antibiotic therapy?
The CDC’s monitoring program, the Antibiotic Use Option of the National Healthcare Safety Network, captures electronic data on antibiotic use in a facility, enabling monitoring of use in each unit. Data can also be aggregated at regional, state, and national levels.
How do we monitor efficacy of antibiotics?
What should you assess after giving antibiotics?
Why is it important to monitor the use of antibiotics?
What are the key nursing considerations in caring for a patient on antibiotics?
What are three possible adverse reactions when giving Gantrisin?
- stomach pain.
- bloating.
- gas.
- headache.
- dizziness.
- ringing in your ears, or.
- swollen, black, or "hairy" tongue.
Which labs should be monitored before and during the antibiotic therapy?
What are the monitored antimicrobials?
What is antibiotic surveillance?
How does an antibiogram help in empiric therapy?
In a November 2014 study published in the journal Infection Control and Hospital Epidemiology, researchers determined that 85% of the prescriptions ordered in skilled nursing facilities were decided upon empirically, but only 35% of those prescriptions were deemed appropriate when compared with the eventual pathogen identification and susceptibility profile obtained from the clinical laboratory. However, in one nursing facility where use of antibiograms was implemented to direct selection of empiric therapy, appropriateness of empiric therapy increased from 32% before antibiogram implementation to 45% after implementation of antibiograms. [1] Although these data are preliminary, they do suggest that health-care facilities can reduce the number of inappropriate prescriptions by using antibiograms to select empiric therapy, thus benefiting patients and minimizing opportunities for antimicrobial resistance to develop.
Why are antibiograms useful?
Antibiograms are useful for monitoring local trends in antimicrobial resistance/susceptibility and for directing appropriate selection of empiric antibacterial therapy.
What is a microdilution tray?
A microdilution tray can also be used to determine MICs of multiple antimicrobial drugs in a single assay. In this example, the drug concentrations increase from left to right and the rows with clindamycin, penicillin, and erythromycin have been indicated to the left of the plate.
What is the lowest dilution in a dilution test?
Figure 1. In a dilution test, the lowest dilution that inhibits turbidity (cloudiness) is the MIC. In this example, the MIC is 8 μg/mL. Broth from samples without turbidity can be inoculated onto plates lacking the antimicrobial drug. The lowest dilution that kills ≥99.9% of the starting inoculum is observed on the plates is the MBC. (credit: modification of work by Suzanne Wakim)
What determines the size of the zone of inhibition?
There are multiple factors that determine the size of a zone of inhibition in this assay, including drug solubility, rate of drug diffusion through agar, the thickness of the agar medium, and the drug concentration impregnated into the disk. Due to a lack of standardization of these factors, interpretation of the Kirby-Bauer disk diffusion assay provides only limited information on susceptibility and resistance to the drugs tested. The assay cannot distinguish between bacteriostatic and bactericidal activities, and differences in zone sizes cannot be used to compare drug potencies or efficacies. Comparison of zone sizes to a standardized chart will only provide information on the antibacterials to which a bacterial pathogen is susceptible or resistant.
Why did Nakry have a UTI?
Nakry’s UTI was likely caused by the catheterizations she had in Vietnam.
Which method can determine the MICs of multiple antimicrobial drugs against a microbial strain using a
The method that can determine the MICs of multiple antimicrobial drugs against a microbial strain using a single agar plate is called the Etest.
How much of antibiotics are unnecessary in the outpatient setting?
At least 30% of antibiotics prescribed in the outpatient setting are unnecessary, meaning that no antibiotic was needed at all. 2. Total inappropriate antibiotic use, inclusive of unnecessary use and inappropriate selection, dosing and duration, may approach 50% of all outpatient antibiotic use. 3, 4, 5. Antibiotic prescribing in the outpatient ...
What is appropriate antibiotic prescribing?
Appropriate antibiotic prescribing means antibiotics are only prescribed when needed, and when needed, the right antibiotic is selected and prescribed at the right dose and for the right duration. Appropriate antibiotic prescribing should be in accordance with evidence-based national and local clinical practice guidelines, when available.
How much of antibiotics are unnecessary?
CDC estimates that at least 30% of antibiotics prescribed in the outpatient setting are unnecessary, meaning that no antibiotic was needed at all. 2 Total inappropriate antibiotic use, inclusive of unnecessary use and inappropriate selection, dosing and duration, may approach 50% of all outpatient antibiotic use. 3, 4, 5.
How many antibiotics were dispensed in 2014?
In 2014, 266.1 million courses of antibiotics are dispensed to outpatients in U.S. community pharmacies. This equates to more than 5 prescriptions written each year for every 6 people in the United States. 1. At least 30% of antibiotics prescribed in the outpatient setting are unnecessary, meaning that no antibiotic was needed at all. 2.
What is the most commonly prescribed antibiotic?
An estimated 80-90% of the volume of human antibiotic use occurs in the outpatient setting. 10, 11. Azithromycin and amoxicillin are among the most commonly prescribed antibiotics. 1.
What age should a child be tested for pharyngitis?
Appropriate testing for children with pharyngitis: percentage of children 2 to 18 years of age who were diagnosed with pharyngitis, prescribed an antibiotic and received a group A Streptococcus (strep) test for the episode.
Can antibiotics be prescribed in outpatient settings?
Antibiotic prescribing in the outpatient setting varies by state. 1
Why is it important to narrow the antibiotic spectrum?
This is a critically important component of antibiotic therapy because it can reduce cost and toxicity and prevent the emergence of antimicrobial resistance in the community. Antimicrobial agents with a narrower spectrum should be directed at the most likely pathogens for the duration of therapy for infections such as community-acquired pneumonia or cellulitis in the ambulatory setting because specific microbiological tests are not typically performed.
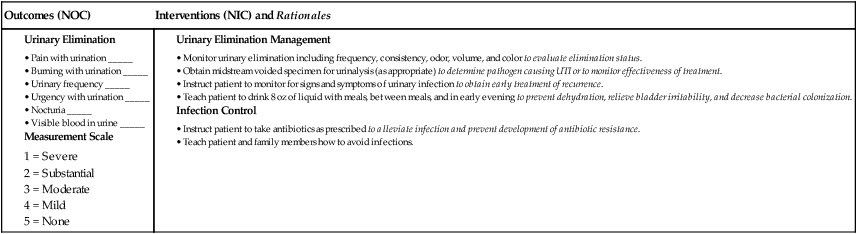
Why is it important to know how well antimicrobials are working?
Renal and Hepatic Function. Because the kidney and the liver are the primary organs responsible for elimination of drugs from the body, it is important to determine how well they are functioning during antimicrobial administration. In most cases, one is concerned with dose reduction to prevent accumulation and toxicity in patients with reduced renal or hepatic function. However, sometimes doses might need to be increased to avoid underdosing young healthy patients with rapid renal elimination or those with rapid hepatic metabolism due to enzyme induction by concomitant use of drugs such as rifampin or phenytoin.
What is antimicrobial therapy?
Antimicrobial agents are some of the most widely, and often injudiciously, used therapeutic drugs worldwide. Important considerations when prescribing antimicrobial therapy include obtaining an accurate diagnosis of infection; understanding the difference between empiric and definitive therapy; identifying opportunities to switch ...
What is AST in microbiology?
When a pathogenic microorganism is identified in clinical cultures, the next step performed in most microbiology laboratories is antimicrobial susceptibility testing (AST). Antimicrobial susceptibility testing measures the ability of a specific organism to grow in the presence of a particular drug in vitro and is performed using guidelines established by the Clinical and Laboratory Standards Institute, 7 a nonprofit global organization that develops laboratory process standards through extensive testing and clinical correlation. The goal of AST is to predict the clinical success or failure of the antibiotic being tested against a particular organism. Data are reported in the form of minimum inhibitory concentration (MIC), which is the lowest concentration of an antibiotic that inhibits visible growth of a microorganism, and are interpreted by the laboratory as “susceptible,” “resistant,” or “intermediate,” according to Clinical and Laboratory Standards Institute criteria. A report of “susceptible” indicates that the isolate is likely to be inhibited by the usually achievable concentration of a particular antimicrobial agent when the recommended dosage is used for the particular site of infection. For this reason, MICs of different agents for a particular organism are not directly comparable. For example, MICs of 1 (susceptible) for ciprofloxacin and 2 (susceptible) for ceftriaxone against Escherichia coli do not imply that ciprofloxacin is twice as active as ceftriaxone. Instead, it indicates that concentrations achieved by giving recommended doses of both drugs are likely to be active against the organism. Although AST results are generally quite useful in narrowing the antibiotic regimen, AST has some limitations that should be kept in mind. First, it is important for both clinicians and laboratory personnel to be aware of the site of infection. For example, an isolate of S aureus could be reported as susceptible to cefazolin in vitro; however, if this particular isolate was obtained from the cerebrospinal fluid (CSF), cefazolin would not be an optimal therapeutic choice because it does not achieve therapeutic concentrations in the CSF. Clinical laboratories may provide different AST interpretations for different sites of infection (eg, meningitis and nonmeningitis AST results for S pneumoniae ). In addition, some organisms carry enzymes that, when expressed in vivo, can inactivate antimicrobial agents to which the organism shows in vitro susceptibility. Although their presence is not immediately apparent from AST results, certain AST “patterns” can provide a clue to their existence. For example, extended-spectrum β-lactamases (ESBLs) in Enterobacteriaceae are enzymes that mediate resistance to almost all β-lactam agents except carbapenems (eg, meropenem or imipenem). Extended-spectrum β-lactamases can be difficult to detect because they have different levels of in vitro activity against various cephalosporins. In clinical practice, susceptibility to cephamycins (cefoxitin, cefotetan) but resistance to a third-generation cephalosporin (eg, cefpodoxime, cefotaxime, ceftriaxone, ceftazidime) or aztreonam should alert one to the possibility of ESBL production. The production of ESBL should also be suspected when treatment with β-lactams fails despite apparent in vitro susceptibility. This should lead to additional testing, which usually involves growing the bacteria in the presence of a third-generation cephalosporin alone and in combination with clavulanic acid (a β-lactamase inhibitor); enhanced bacterial inhibition with the addition of clavulanic acid indicates ESBL. When detected by the laboratory, these bacteria should be considered resistant to all β-lactam agents except the carbapenem class.
How to extend the antimicrobial spectrum?
To Extend the Antimicrobial Spectrum Beyond That Achieved by Use of a Single Agent for Treatment of Polymicrobial Infections. When infections are thought to be caused by more than one organism, a combination regimen may be preferred because it would extend the antimicrobial spectrum beyond that achieved by a single agent. For example, most intra-abdominal infections are usually caused by multiple organisms with a variety of gram-positive cocci, gram-negative bacilli, and anaerobes. Antimicrobial combinations, such as a third-generation cephalosporin or a fluoroquinolone plus metronidazole, can be used as a potential treatment option in these cases and can sometimes be more cost-effective than a comparable single agent (eg, a carbapenem).
Why are gram positive bacteria endemic?
They are commonly caused by drug-resistant organisms, both gram-positive (eg, methicillin-resistant Staphylococcus aureus[MRSA]) and gram-negative (eg, Pseudomonas aeruginosa) bacteria, which are often endemic in hospitals because of the selection pressure from antimicrobial use.
When critically ill patients require empiric therapy?
When Critically Ill Patients Require Empiric Therapy Before Microbiological Etiology and/or Antimicrobial Susceptibility Can Be Determined. As already discussed, antibiotic combinations are used in empiric therapy for health care–associated infections that are frequently caused by bacteria resistant to multiple antibiotics. Combination therapy is used in this setting to ensure that at least 1 of the administered antimicrobial agents will be active against the suspected organism (s). For example, when a patient who has been hospitalized for several weeks develops septic shock and blood cultures are reported to be growing gram-negative bacilli, it would be appropriate to provide initial therapy with 2 agents that have activity against gram-negative bacilli, particularly P aeruginosa, which is both a common nosocomial pathogen and frequently resistant to multiple agents—in this case, a combination of an antipseudomonal β-lactam with a fluoroquinolone or aminoglycoside could be used.
Why is it important to improve antibiotics?
Improving our antibiotic use is critical to the safety of our patients and the future of medicine. This can improve patient outcomes, save money, reduce resistance, and help prevent negative consequences such as Clostridium difficileinfection. The US Centers for Disease Control and Prevention (CDC) is undertaking a nationwide effort to appropriately improve antibiotic use in inpatient and outpatient settings.
How much antibiotics are unnecessary?
One study of hospitalized patients not in the intensive care unit found that 30% of 1,941 days of prescribed antimicrobial therapy were unne cessary, mostly because patients received antibiotics for longer than needed or because antibiotics were used to treat noninfectious syndromes or colonizing microorganisms.5
How often are antibiotics prescribed in hospitals?
Antibiotics are probably the most frequently prescribed drugs in US hospitals. Data from 2006 to 2012 showed that 55% of hospitalized patients received at least 1 dose of an antibiotic and that overall about 75% of all hospital days involved an antibiotic.4Rates did not vary by hospital size, but nonteaching hospitals tended to use antibiotics more than teaching hospitals. Antibiotic use is much more common in intensive care units than in hospital wards (1,092 and 720 days of antibiotic treatment per 1,000 patient-days, respectively).
How much did antibiotic use drop?
Although overall antibiotic use did not change significantly over the years of the survey, use patterns did: fluoroquinolone use dropped by 20%, possibly reflecting rising resistance or increased attention to associated side effects (although fluoroquinolones remain the most widely prescribed inpatient antibiotic class), and use of first-generation cephalosporins fell by 7%. A cause for concern is that the use of broad-spectrum and “last-resort” antibiotics increased: carbapenem use by 37%, vancomycin use by 32%, beta-lactam/beta-lactamase inhibitor use by 26%, and third- and fourth-generation cephalosporin use by 12%.4
What is the goal of antibiotic stewardship?
The primary goal of antibiotic stewardship is better patient care . The goal is not reduced antibiotic use or cost savings, although these could be viewed as favorable side effects. Sometimes, better patient care involves using more antibiotics: eg, a patient with presumed sepsis should be started quickly on broad-spectrum antibiotics, an action that also falls under antibiotic stewardship. The focus for stewardship efforts should be on optimizing appropriate use, ie, promoting the use of the right agent at the correct dosage and for the proper duration.
How long after antibiotics can you get C difficile?
The risk of C difficileinfection is 7 to 10 times higher than at baseline for 1 month after antibiotic use and 3 times higher than baseline in the 2 months after that.11Multiple studies have found that stewardship efforts to reduce antibiotic use have resulted in fewer C difficileinfections.
Why is research important?
Research to better understand the problem and how to fight it
What are the symptoms of antibiotics?
As the body tries to rid itself of bacteria, classic signs of inflammation (e.g. swelling, heat, redness, and pain), fever, and lethargy begin to show up. The goal of antibiotic therapy is to decrease the population of invading bacteria to a point at which the human immune system can effectively deal with the invader.
What is the class of antibiotics that are effective against Gram positive and Gram negative bacteria?
Carbapenems are a relatively new class of broad-spectrum antibiotics effective against gram-positive and gram-negative bacteria.
What is the antibiotic used for tuberculosis?
Antimycobacterials. Antimycobacterials are antibiotics used in the treatment of infections caused by pathogens responsible for tuberculosis and leprosy. Mycobacterium tuberculosis causes tuberculosis, the leading cause of death from infectious disease in the world.
What is a tetracycline?
Tetracyclines are semisynthetic antibiotics based on the structure of a common soil mold.
What was the first antibiotic?
Penicillin was the first antibiotic introduced for clinical use. Various modifications were subsequently made to address resistant strains and to decrease drug adverse effects. Penicillinase-resistant antibiotics were developed to address penicillin-resistant bacteria.
What is an antibiotic 2021?
ADVERTISEMENTS. Antibiotics are agents made from living microorganisms, synthetic manufacturing, and genetic engineering that are used to inhibit specific bacteria. They can be bacteriostatic, bactericidal, or both.
Can antibiotics cure common colds?
Adults. This age group has the tendency to cure simple manifestations with antibiotics. Therefore, it is important to educate them that antibiotics are effective only for certain bacteria and not for simple manifestations like common colds, which may be viral.
What is non standard method of measuring antibiotic use and resistance?
A non standard method of measuring antibiotic use and resistance presents challenges in evaluating epidemiologic studies against one another. Ignoring inappropriate use of antibiotics can lead to unnecessarily high rates of resistance in the healthcare setting.
What is the utility of antibiograms?
Pakyz, AL. “The Utility of Hospital Antibiograms as Tools for Guiding Empiric Therapy and Tracking Resistance”. Pharmacotherapy. vol. 27. 2007. pp. 1306-12.
What is an antibiogram?
Antibiograms report the proportion of resistant isolates to a given antimicrobial agent. Changes in antimicrobial use itself can alter the number of susceptible isolates without actually changing the number of resistant isolates. Here a researcher could misinterpret changes in the proportion of resistance as a change in the burden of resistance.
What is the purpose of antimicrobial stewardship?
The Infectious Disease Society of America (IDSA) and Society of Healthcare Epidemiologists (SHEA) have promoted better antimicrobial stewardship as a method for reducing antimicrobial resistance. Antimicrobial stewardship and infection control programs share the common goal of managing antimicrobial resistance in order to improve patient healthcare and outcomes. The ability to measure antimicrobial use and resistance appropriately are crucial pieces of information in evaluating such programs. A number of metrics are available for quantifying use and resistance, and practitioners must use caution to understand the implications of each.
What happens if few isolates of a given species are tested?
If few isolates of a given species are tested, then the proportion of resistance may not reflect the true level of resistance in the population. Researchers must fully understand the limitations of the metric being chosen for measuring antimicrobial use.
When measuring antimicrobial resistance, if antibiograms are constructed ignoring CLSI guidelines?
When measuring antimicrobial resistance, if antibiograms are constructed ignoring CLSI guidelines (e.g., duplicate isolates are included, surveillance isolates are included) then the proportion of resistance reported may actually be inflated. If few isolates of a given species are tested, then the proportion of resistance may not reflect ...
Can antimicrobial resistance be inflated?
Several studies have identified deficiencies in adherence to standard methodologies for measuring antibiotic resistance through antibiograms. When measuring antimicrobial resistance, if antibiograms are constructed ignoring CLSI guidelines (e.g., duplicate isolates are included, surveillance isolates are included) then the proportion of resistance reported may actually be inflated. If few isolates of a given species are tested, then the proportion of resistance may not reflect the true level of resistance in the population.
Why should psychologists learn more about psychopharmacology?
While health psychologists, given their specialty, obviously must receive more education and training in how all medications affect patients, all psychologists should learn more about psychopharmacology because teasing apart potential medication problems and helping patients manage their regimens can significantly improve care , says clinical psychologist Virginia Waters, PhD, who has private practices in New Jersey and New York. “We are often the most stable medical caregiver for our clients and the one they speak with the most often,” she says. “As a result, our patients look to us for guidance when things are not adequately explained to them.”
How many people take psychiatric medications?
Many of these medications are psychotropics: Research suggests that over the course of a year, one in six American adults takes at least one psychiatric drug, including antidepressants, anti-anxiety medications and antipsychotics ( JAMA Internal Medicine, Vol. 177, No. 2, 2017).
What is the Coons test?
During the initial visit, Coons asks all patients to complete standardized measures to assess symptoms and severity of mood, anxiety, fatigue, pain, post-traumatic stress disorder and sexual functioning and conducts a clinical interview to evaluate how a patient’s medications may—or may not—be working.