How do you increase the pH level?
Pure or distilled water has a pH level of 7, which means it is neutral. If you want to increase the pH of water, you must add an alkaline substance, such as baking powder, to it. If you want to decrease the pH of water, you add an acidic substance, such as lemon juice, to it.
How do you adjust pH in wastewater treatment?
To lower pH acids such as sulfuric acid (H2SO4) can be used. A newer technology is to use carbon dioxide to adjust pH without the chance to over shoot your target. The correct choice all stems from how much adjustment is required. Raising pH is usually done using sodium hydroxide (caustic soda) (NaOH).
How do you increase the pH of water naturally?
If the pH of water is low in that case soda ash or sodium bicarbonate can be added to water to increase pH value. Naturally, we can also do it by adding clean rocks or quartz porphyry to the drinking water to increase pH. On the other hand, citric acid or vinegar can be added to decrease pH value of water.
What causes low pH in wastewater?
If the influent pH is satisfactory, then the low effluent pH is usually caused by nitrification in combination with low natural alkalinity in the wastewater. If ammonia removal is required, then nitrification must continue.
What can be added to a solution to control the pH?
For buffers in acid regions, the pH may be adjusted to a desired value by adding a strong acid such as hydrochloric acid to the particular buffering agent. For alkaline buffers, a strong base such as sodium hydroxide may be added.
How do you fix low pH well water?
If your water is acidic (low pH), you can use a neutralizing filter containing calcite or ground limestone (calcium carbonate) or magnesia (magnesium oxide) to raise the pH. Neutralizing filters must be backwashed periodically since they serve as mechanical filters to remove solid particles from the water. !
What is the most common method of correcting the low pH?
pH Correction and Acid Neutralization The two most common practices to do this are: Passing the acidic water through a bed of neutralizing media (i.e. calcite or magnesium oxide). Feeding a liquid chemical solution directly into the water (i.e. soda ash injection).
What should be added to increase the pH value?
To increase the pH value of a given neutral solution an alkali should be added as an alkaline solution that has greater pH than that of an acidic solution. For example, to increase the pH of a given neutral solution sodium hydroxide, potassium hydroxide solutions are added.
Why is my well water acidic?
Drinking water pH can be affected by chemicals and mineral ions in the water supply. In the US, you’re most likely to find acidic water in Oregon,...
Is acidic well water safe to drink?
It depends on how acidic the water is, and the metal contaminants it is exposed to. Acid water that contains metal ions like copper, lead and arsen...
What is the average pH of well water?
Most surface water wells have a pH range of 6.5 to 8.5. Groundwater systems have an average pH range of 6 to 8.5. Remember that this is just the av...
Is there a problem with high pH?
You might think that the higher the pH, the better, when it comes to the quality of your drinking water. But actually, alkaline water can be just a...
Can acid neutralizers or soda ash injection systems also filter water?
No. Acid neutralizers that use corosex may be able to remove a small amount of iron, but for general filtration, you’ll need to install a whole hom...
What happens if you drink water with a pH of 7?
Water with a value greater than 7 indicates alkalinity and tends to affect the taste of the water. Alkaline drinking water may take on a “soda” taste. Corrosion problems also can occur in plumbing.
What is the pH of water?
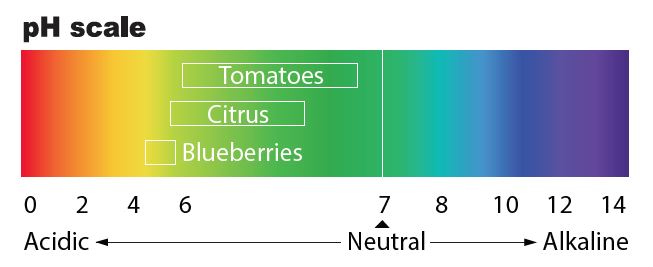
pH is an indicator of the acid or alkaline condition of water. The pH scale ranges from 0-14; 7 indicates the neutral point. The normal pH range of drinking water is 6 – 8.5. The pH is mostly a result of natural geological conditions at the site and the type of minerals found in the local rock. The pH can also be affected by acid rain.
How does soda ash work?
How soda ash/sodium hydroxide injection works. This treatment method is used if water is acidic (low pH). Soda ash (sodium carbonate) and sodium hydroxide raise the pH of water to near neutral when injected into a water system. Unlike neutralizing filters, they do not cause hardness problems in treated water.
How does a neutralizing filter work?
How neutralizing filters work. A neutralizing filter is used if drinking water is acidic (low pH). It is a simple treatment device that raises the pH of water by adding a neutralizing material. However, it should be noted that the neutralization process may increase water hardness.
What does acid injection do to water?
Acid injection treats water with a high pH by lowering the pH of water to around 7, which eliminates the soda taste and can improve the effectiveness of chlorination. This method also reduces the potential of pipe corrosion as water with a pH above 9 can corrode metals such as brass, copper, zinc, aluminum and iron.
Why install a cartridge filter before neutralizing?
Installing a cartridge filter prior to the neutralizing filter will remove solid particles from the water and can help to prolong the life of the neutralizing filter.
How to store sodium hydroxide?
Use caution if using sodium hydroxide. If adding it manually, maintain good ventilation to avoid breathing vapors. Add the chemical slowly to the water and ensure complete mixing. Be sure to wear protective gloves, goggles and clothing to avoid skin and eye contact with the chemical. Store sodium hydroxide in a cool, dry place away from flammable materials.
How to increase pH in fish tank?
Fill a mesh bag with crushed coral for a simple way to increase pH. Use ½ cup (200 g) of crushed coral for every 20 gallons (76 L) in your aquarium to increase the pH. Rinse the coral off in your sink before transferring it into a small mesh bag. Set the bag in your fish tank where there is a current, such as next to a filter. Wait 2-3 days before checking the pH of your aquarium again and add more crushed coral to the bag until you reach the desired pH level.
How to test pH of water?
1. Test the pH of your water with test strips. Dip the end of a paper strip or the pH meter into your water. The pH paper will start to change colors based on the acidity of your water. Match the colors to those provided on the guide to determine the pH of your water. pH test strips can be purchased online.
What is the difference between alkalinity and pH?
pH measures the acidity of the water while alkalinity is the measurement of water’s ability to resist pH change. Alkalinity also affects how clear or cloudy your pool appears.
How to make your aquarium more concentrated?
Make sure your aquarium has a filter so the water can move through the limestone and spread the sediment. Otherwise, your aquarium will become concentrated in different areas.
What is neutralizing water filter?
Neutralizing filters contain calcite or magnesium oxide and connect directly to your water line . As the water passes through the filter, the calcite raises the pH levels of your water. Call a plumber to attach the filter to your water line. Neutralizing filters work well for raising the pH of well water.
How to make water alkaline?
Mix baking soda into a serving of water to change the pH and alkalinity. Pour yourself 1 cup (240 ml) of water and pour in 1 tsp (4 g) of baking soda to raise the pH by 1. Stir the solution together thoroughly to bump up your pH levels to make alkaline water.
How to measure pH in aquarium?
1. Use a pH meter to accurately measure your aquarium’s pH levels. Dip the end of your pH meter into your aquarium. Keep the meter submerged until the numbers on the screen stabilize. Once you remove the meter from your aquarium, rinse the end of the meter with distilled water to clean it.
How to raise pH in water?
Increasing pH with Neutralizing Filters. Neutralizing filters, which increase pH values through the addition of neutralizing materials, can also be effective for raising the pH of drinking water. These filters typically contain calcium carbonate or similar substances, which dissolve as they interact with untreated water.
What is the recommended pH level for drinking water?
In fact, failing to measure existing drinking water values may lead to an excessive increase in pH beyond the current recommendation of 6.5 to 8.5.
What is the pH of water?
The Basics About pH. The pH scale -- which ranges from 0 to 14 -- measures the acidity and alkalinity of various substances. While pure, unadulterated water is generally considered to have a neutral pH of 7, the addition of various compounds can cause it to become more acidic or alkaline.
What minerals affect the pH of water?
In fact, minerals that are naturally found underground -- including calcium, magnesium and iron, among others -- may affect the pH of water that is used for human consumption. Mechanical problems, such as faulty plumbing,can also change the pH of your drinking water. Advertisement.
Why is water important?
Image Credit: luchschen/iStock/Getty Images. Water is often touted as a necessity for individuals who are interested in maintaining good health and promoting optimal physiological function. However, while the amount of water that you drink is important, its quality is just as crucial. In fact, water that has a pH outside the range ...
Can you use soda ash in water softener?
Because it can increase the sodium in drinking water, soda ash is not recommended for use by individuals who are on low-sodium diets.
How to increase the pH of pool water?
You can increase the pH of your pool water in simple 5 steps. Let’s go through this process step by step. Step-1. Test the pH level of pool water. This is a very important step before you take any action to increase the pH of pool water. You should always use the best pool water test kit for pool to check & ensure the pH level.
What is the best way to raise pH in a pool?
Prefer Soda Ash (Sodium Carbonate) if you want to raise pH in the pool without raising alkalinity. Note: Soda ash (sodium carbonate) increases the pH and slightly increase the alkalinity while, baking soda (sodium bicarbonate) increase more alkalinity and slightly increase the pH. Remember this thumb rule, 5.3 ounces of soda ash is required ...
What is the most common disinfectant for pool water?
pH balance is the most important parameter for the proper working of disinfectant chemicals. The most popular & widely used disinfectant for pool water is chlorine.
Why is my pool water pH low?
Low pH can create many negative effects like corrosion of pool ladders, liners & other pool components. Swimmers are also feeling the effects like irritation on the skin and burning eyes etc due to low pool water pH. The major cause for low pH in the pool is improper use of chemicals, overuse of pool & heavy rainfall.
Why is my pH low in my pool?
The major cause for low pH in the pool is improper use of chemicals, overuse of pool & heavy rainfall.
What should the pH of a pool be?
If your pool water pH is below 7.2 then you must take an action to raise the pH of pool water.
What happens if your pool water pH is low?
If your pool water pH is low then it can cause many serious issues like irritation, sickness, burning of eyes, etc.
What is the best acid to neutralize pH?
Sulfuric acid and sodium hydroxide (caustic) are most commonly used for neutralizing acids or bases. Caution must be used for pH adjust applications as an exothermic reaction will occur generating heat. The more severe the application the more heat generated. For example; pH adjusting tap water will create very little heat and is a non-issue. Neutralizing solutions with a high percentage acidic or bases can generate significant heat and therefore must be considered in design and materials. Use caution when doing any pH adjustment.
What is a pH adjuster?
A pH adjuster is a chemical used to alter the pH or Potential Hydrogen level. pH (Potential Hydrogen) is the measurement of the activity of the hydrogen ion or how basic or acidic something is. By adding a pH reagent such as an acid you can drive pH downward.
Can you use pH adjusters in water treatment?
pH Adjustment in Water Treatment. Unfortunately in the world of pH there is no one size fits all standard for what to use for pH adjustments in water treatment. pH Adjusters for Water Treatment include many chemicals that have varying benefits and disadvantages. We suggest speaking directly with one of our chemists to analyze your application ...
What happens when the pH of a boiler drops below 8.5?
When the boiler water pH drops below about 8.5, a corrosion called acid attack can occur. The effect exhibits rough pitted surfaces. The presence of iron oxide deposits on boiler surfaces can encourage this kind of corrosion.
What is the caustic concentration of water in a boiler?
Its caustic concentration can be as high as 10,000-100,000 ppm. Careful control of boiler water chemistry can prevent caustic gouging. If the “free hydroxide alkalinity”.
What can prevent caustic gouging?
Careful control of boiler water chemistry can prevent caustic gouging. If the “free hydroxide alkalinity”
Is water acidic or basic?
Pure water is neutral, with a pH of 7.0. When chemicals are mixed with water, the mixture can become either acidic or basic (alkaline). Vinegar and lemon juice are acidic substances, while laundry detergents and ammonia are basic (alkaline). Chemicals that are very basic or very acidic are called “reactive.”.
Is a pH of 7 acidic?
A pH of 7 is neutral. A pH less than 7 is acidic, and a pH greater than 7 is basic (alkaline). Each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times (10 times 10) more acidic than a pH of 6.
Is H2O an acid or base?
H2O can act as an acid or a base because it auto-ionizes itself, meaning it gives protons back and forth within itself, thus acting as both an acid and a base.
Is acidic water corrosive?
Acidic water is corrosive. Alkalinic water is more prone to scaling. Alkalinity is a measure of the bicarbonate (HCO3), carbonate (CO3) and hydroxyl (OH) ions in the water. pH and alkalinity ratings are NOT the same and are NOT proportional. pH is rated on the Scale and alkalinity is measured in parts per million (ppm).
How to raise pH in well water?
One of the most convenient methods to raise well water pH, hardness, and alkalinity is to use a calcite neutralizer filter. These filters will typically raise the pH of the well water from 7.0 to 8.0 and add 30 to 100 ppm of hardness depending on the alkalinity and water hardness.
What pH should a calcite tank be?
Calcite Neutralizer tanks with natural crushed and screened pure calcium carbonate easily neutralize acidic waters from 6.0 to 6.9. Below 6.0 a blend of calcite and ‘Corosex©” is recommended.
How does calcite work?
The calcite and calcite-blend neutralizers work by adding calcium to the water, and it will increase the calcium hardness of the water, making the water ‘harder’. However, most acidic well water is soft to begin with and after passing through the neutralizer will be harder, but not hard enough to warrant a water softener.
What causes corrosion in water pipes?
On private water systems, one of the most common causes of pipe and fixture corrosion is from low pH, which can be defined as acidic water with a pH of less than 7.0 pH. Signs of acid water are corrosion of fixtures, blue staining (from copper pipes) or rust staining (from iron pipes). Common causes for acidic water are acid rainfall due ...
What causes acidic water?
Common causes for acidic water are acid rainfall due to atmospheric carbon dioxide and other airborne pollutants, runoff from mining spoils and decomposition of plant materials. Corrosion is a natural process involving chemical or electrical degradation of metals in contact with water.
How many parameters are needed for well water?
These 12 parameters are a must-know for all well water owners and are designed to give you results accurately and rapidly.
Is a downflow neutralizer good for a well?
Both upflow and downflow neutralizers are used, but generally, downflow neutralizers that have a periodic automatic backwash are much easier to maintain and tend to work better for residential well water systems.
What is the pH of water?
Under the EPA's drinking water standards, there is no primary standard for pH, but it is recommended that drinking water pH fall between 6.5 and 8.5. Within the water treatment industry, the goal is typically to achieve a pH value of around 7.5 for corrosion control and prevention.
What are the factors that affect pH?
Other influencing factors on pH correction include total hardness, TDS (total dissolved solids), sulfates, and chlorides. Hardness, TDS and pH Correction. Total hardness and Total Dissolved Solids ( TDS) ...
What is the name of the neutralizing media that passes acidic water through?
Passing the acidic water through a bed of neutralizing media (i.e. calcite or magnesium oxide).
What does higher alkalinity mean in water?
A higher alkalinity results in a greater capacity of the water to resist changes in pH from the addition of acids. pH Correction and Acid Neutralization. More often than not, the large majority of pH correction issues in the water treatment industry involve neutralizing acidic pH conditions or raising the pH to above 7.0.
What does pH correction mean?
Acid Neutralizers and pH Correction. What is pH? The pH of your drinking water reflects how acidic or alkaline it is. pH stands for "potential of hydrogen", referring to the amount of hydrogen present in water, and it is measured on a logarithmic scale between 0 and 14.
What is the measure of the water's ability to buffer itself or neutralize acids?
Alkalinity is a measure of the water's quantitative ability to buffer itself or neutralize acids. Total alkalinity is a product of the total sum of carbonate (CO3), bicarbonate (HCO3), and hydroxide (OH) ions present in solution. A higher alkalinity results in a greater capacity of the water to resist changes in pH from the addition of acids.
What are the most acidic water conditions in the United States?
The most common regions for acidic water conditions in the United States are New England, the Mid-Atlantic, and the Pacific Northwest. Even within these regions, the pH and water chemistry can vary widely. The main influence on low pH in these regions is free carbon dioxide, mineral acids, and the lack of sufficient bicarbonate alkalinity.
What acid is used to lower pH?
Now for pH adjustment - most systems use strong chemistry: To lower pH acids such as sulfuric acid (H2SO4) can be used. A newer technology is to use carbon dioxide to adjust pH without the chance to over shoot your target. The correct choice all stems from how much adjustment is required.
What is buffering pH?
Buffering refers to how the pH tends to remain stable once adjusted. Organisms such as AOB & Nitrite Oxidizing Bacteria (NOB) like a slightly alkaline pH while also consuming significant alkalinity (usually expressed as calcium carbonate). Additional alkalinity is required to buffer against organic acids, carbon dioxides (from respiration), ...
What is the pH range of bioaugmentation?
Biological wastewater treatment usually works best in a pH range from 7.0 - 8.0. Remember that this is the "best" range in a general sense. In making bioaugmentation products, we have used strains with pH ranges from 3.0 (Thiobacillus) to 11.0 (alkanophilic Bacillus). The most pH sensitive process tends to be ammonia removal or nitrification.
What pH does sodium bicarbonate have?
Sodium bicarbonate - soluble, tends to max out pH at 8.3 - so low overdosing potential. So remember that you have choices in adjusting pH and buffering the system. Alkalinity or buffering capacity is a key consideration in wastewater treatment especially if you require ammonia oxidation.
What is the pH of ammonia?
The most pH sensitive process tends to be ammonia removal or nitrification. Ammonia Oxidizing Bacteria (AOB) do best at a pH of 7.2 - 8.2 where the free ammonia (NH3-N) is present but is still soluble in water.
Which is more soluble, magnesium hydroxide or sodium carbonate?
Magnesium hydroxide - effective but also has solubility issues. Sodium carbonate - much more soluble, but can be "too strong" a base.
Does sodium hydroxide raise pH?
Raising pH is usually done using sodium hydroxide (caustic soda) (NaOH). As with sulfuric acid, sodium hydroxide immediately raises pH. It does not buffer the solution & buffering is a key concept that we need to consider in biological systems. Buffering refers to how the pH tends to remain stable once adjusted.