Precautions
Probuphine has been assigned a specific J-Code for Probuphine (J0570) beginning January 1, 2017. This for the drug code only. The Medicare Allowable is per implant (descriptor 74.2mg) and reimbursed at $1,311.75 which is ASP + 6%.
What is the J-code for Probuphine?
The PROBUPHINE implants are placed just under the skin of the inside of your upper arm through a minor surgical procedure. The implants are soft, flexible, and about the size of a matchstick. Your healthcare provider will cover the site where PROBUPHINE was inserted with 2 bandages.
What is the Probuphine procedure?
PROBUPHINE is a controlled substance (CIII) because it contains buprenorphine that can be a target for people who abuse prescription medicines or street drugs. If it comes out of your arm, keep the implant in a safe and secure place away from others, especially children.
Is Probuphine a controlled substance?
PROBUPHINE contains a medicine called buprenorphine. Buprenorphine is an opioid that can cause serious and life-threatening breathing problems, especially if you take or use certain other medicines or drugs. Call your healthcare provider right away or get emergency help if you: These can be signs of an overdose or other serious problems.
What should I know about Probuphine before taking it?

What is Probuphine implant?
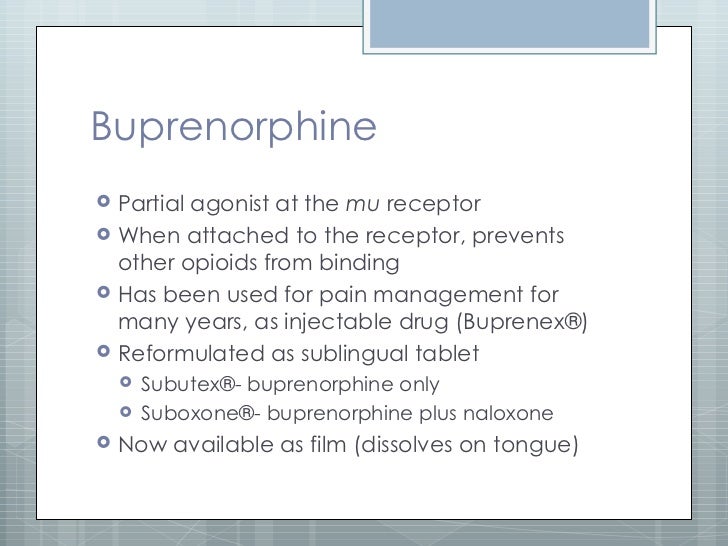
Implantable buprenorphine (Probuphine) is an implantable partial opioid agonist. It is labeled for the treatment of patients with opioid use disorder who have achieved and sustained clinical stability on a transmucosal buprenorphine product at low to moderate dosages, defined as no more than 8 mg per day.
How do you bill buprenorphine?
Example: In the case of a patient getting injectable buprenorphine, OTPs bill HCPCS code G2069 for the week you supply the injection. For the following weeks, when you supply at least 1 non-drug service, bill HCPCS code G2074, which describes a non-drug bundle.
What is the CPT code for buprenorphine?
There are no specific CPT codes for buprenorphine therapy. Most reported services directly related to buprenorphine therapy will be Evaluation and Management services (99201-99205, 99211-99215) or Pharmacologic Management (90862) if rendered by a psychiatrist.
What is the J code for Sublocade?
HCPCS Code for Injection, buprenorphine extended-release (sublocade), greater than 100 mg Q9992.
What drug class is buprenorphine?
Buprenorphine is a schedule III narcotic analgesic. It was first marketed in the United States in 1985 as a schedule V narcotic analgesic.
What schedule is buprenorphine?
Opioids that fall under Schedule III, IV, and V are approved for medical treatment in the United States and have a lower risk for abuse and psychological or physical dependence. Some opioids in this group include: Buprenorphine, and all drugs containing Buprenorphine, like Suboxone, is a Schedule III drug.
How do you bill for opioid treatment?
HCPCS code G2080 may be billed when counseling or therapy services are furnished that substantially exceed the amount specified in the patient's individualized treatment plan. OTPs are required to document the medical necessity for these services in the patient's medical record.
What is the J code for Suboxone?
HCPCS Code for Buprenorphine/naloxone, oral, greater than 10 mg buprenorphine J0575.
What is CPT H0020?
Providers should bill one unit of Healthcare Common Procedure Coding System (HCPCS) code H0020 – Alcohol and/ or drug services; methadone administration and/or service (provision of the drug by a licensed program), for each day a member presents for treatment.
What is CPT code 96372 used for?
CPT® code 96372: Injection of drug/substance under skin or into muscle | American Medical Association.
What is Q9992?
Q9992 is a valid 2022 HCPCS code for Injection, buprenorphine extended-release (sublocade), greater than 100 mg or just “Buprenorphine xr over 100 mg” for short, used in Medical care.
Does Medicare cover Sublocade?
Sublocade is usually not covered under Medicare Part D and Medicare Advantage prescription drug plans, but is covered under Medicare Part B as a medical benefit.
What is the J code for buprenorphine?
today announced that the Centers for Medicare & Medicaid Services (CMS) has granted a Healthcare Common Procedure Coding System (HCPCS) code, or permanent J-code, for Probuphine, the first and only six-month buprenorphine implant for the maintenance treatment of opioid addiction. The new J-code (J0570) became effective January 1, 2017 and coincides with the activation of a new field force to drive the next phase of Probuphine adoption.
What is Braeburn Pharmaceuticals?
Braeburn Pharmaceuticals, an Apple Tree Partners company, is a commercial-stage pharmaceutical company delivering individualized medicine in neuroscience. Long-acting therapeutic treatment options can be essential to improving patient outcomes and facilitating recovery in neurological and psychiatric disorders, which are often complicated by stigma and present significant public health challenges. Braeburn's commercial product, Probuphine ® (buprenorphine) implant was approved by the FDA in May 2016. Braeburn's investigational product pipeline consists of long-acting implantable and injectable therapies for serious neurological and psychiatric disorders, including opioid addiction, pain, and schizophrenia. Braeburn's pipeline products are at various stages of clinical development and include weekly and monthly CAM2038, subcutaneous injection depot formulations of buprenorphine, being investigated in opioid addiction and pain, BB0417 buprenorphine/granisetron injectable for acute pain, and BB0817, six-month risperidone implant being investigated in schizophrenia. More information on Braeburn can be found at www.braeburnpharmaceuticals.com.
Is probuphene available for insertion?
Because of the risks associated with insertion and removal, PROBUPHINE is available only through a restricted program called the PROBUPHINE REMS Program. All Healthcare Providers must successfully complete a live training program on the insertion and removal procedures and become certified, prior to performing insertions or prescribing PROBUPHINE implants. Patients must be monitored to ensure that PROBUPHINE is removed by a healthcare provider certified to perform insertions.
What is level 2 of HCPCS?
The HCPCS Level II Code Set is one of the standard code sets used for medical claims processing of office administered drugs. The HCPCS is divided into two principal subsystems, referred to as level I and level II of the HCPCS. Level I of the HCPCS is comprised of CPT® (Current Procedural Terminology), a numeric coding system maintained by the American Medical Association (AMA). The CPT® is a uniform coding system consisting of descriptive terms and identifying codes that are used primarily to identify medical services and procedures furnished by physicians and other health care professionals. These health care professionals use the CPT® to identify services and procedures for which they bill public or private health insurance programs. Decisions regarding the addition, deletion, or revision of CPT® codes are made by the AMA. The CPT® codes are republished and updated annually by the AMA. Level I of the HCPCS, the CPT® codes, does not include codes needed to separately report medical items or services that are regularly billed by suppliers other than physicians.
Is buprenorphine a partial opioid agonist?
PROBUPHINE contains buprenorphi ne, a partial opioid agonist. Probuphine is indicated for the maintenance treatment of opioid dependence in patients who have achieved and sustained prolonged clinical stability on low-to-moderate doses of a transmucosal buprenorphine-containing product (i.e., doses of no more than 8 mg per day of Subutexor Suboxonesublingual tablet equivalent or generic equivalent).
How much buprenorphine is in IV?
Buprenorphine in IV (2, 4, 8, 12, and 16 mg) and sublingual (12 mg) doses has been administered to opioid-experienced subjects who were not physically dependent, to examine cardiovascular, respiratory, and subjective effects at doses comparable to those used for treatment of opioid dependence.
How long is a buprenorphine implant?
PROBUPHINE ( buprenorphine) implant is a sterile, single, off-white, soft, flexible rod -shaped drug product. It is 26 mm in length and 2.5 mm in diameter. Each implant contains 74.2 mg buprenorphine (equivalent to 80 mg buprenorphine hydrochloride) and ethylene vinyl acetate (EVA). PROBUPHINE is designed to be implanted subdermally by a trained medical professional and to provide sustained delivery of buprenorphine for up to six months.
Can buprenorphine be adjusted during pregnancy?
Dose adjustments of buprenorphine may be required during pregnancy, even if the patient was maintained on a stable dose prior to pregnancy. Withdrawal signs and symptoms should be monitored closely and the dose adjusted as necessary.
Does buprenorphine cause withdrawal symptoms?
Because of the partial opioid agonist properties of buprenorphine, buprenorphine may precipitate opioid withdrawal signs and symptoms in persons who are currently physically dependent on full opioid agonists such as heroin, morphine, or methadone before the effects of the full opioid agonist have subsided. Verify that patients are clinically stable on transmucosal buprenorphine and not dependent on full agonists before inserting PROBUPHINE.
Can buprenorphine cause an allergic reaction?
Allergic reactions to buprenorphine and/or EVA are possible. Cases of hypersensitivity to sublingual buprenorphine have been reported both in clinical trials and in the post-marketing experience. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. The most common signs and symptoms include rashes, hives, and pruritus. A history of hypersensitivity to buprenorphine or EVA is a contraindication to PROBUPHINE use.
Is buprenorphine a partial agonist?
Buprenorphine is a partial agonist at the mu-opioid receptor and chronic administration produces physical dependence of the opioid type, characterized by withdrawal signs and symptoms upon abrupt discontinuation or rapid taper. The withdrawal syndrome is milder than that seen with full agonists, and may be delayed in onset [see Drug Abuse And Dependence ]. If PROBUPHINE implants are not to be immediately replaced upon removal, maintain patients on their previous dosage of sublingual buprenorphine until PROBUPHINE treatment is resumed [see DOSAGE AND ADMINISTRATION ]. Patients who elect to discontinue PROBUPHINE treatment should be monitored for withdrawal with consideration given to use of a tapering dose of transmucosal buprenorphine.
Is buprenorphine a benzodiazepines?
Many, but not all, post-marketing reports regarding coma and death involved misuse by self-injection or were associated with the concomitant use of buprenorphine and benzodiazepines or other CNS depressants, including alcohol.
Who can prescribe, insert, and remove probuphine?
Healthcare Providers Who Prescribe, Insert, and Remove Probuphine. Healthcare providers who prescribe, insert and remove Probuphine surgical procedures in a dual role shall be specially certified. To become certified to prescribe and perform Probuphine surgical procedures, healthcare providers shall: Review the Prescribing Information ...
Who must be certified to insert probuphine?
Healthcare providers who perform Probuphine Surgical procedures must be specially certified. To become certified to insert Probuphine in the Probuphine REMS Program, healthcare providers must: Review the Prescribing Information for Probuphine.
How long is a buprenorphine implant?
Probuphine (buprenorphine) implant is a sterile, single, off-white, soft, flexible rod-shaped drug product. It is 26 mm in length and 2.5 mm in diameter . Each implant contains 74.2 mg buprenorphine (equivalent to 80 mg buprenorphine hydrochloride) and ethylene vinyl acetate (EVA). Probuphine is designed to be implanted subdermally by a trained medical professional and to provide sustained delivery of buprenorphine for up to six months.
Does Medicare cover Sublocade?
Medicare does not have a National Coverage Determination (NCD) specific to the use of Probuphine® and Sublocade® for treatment of moderate to severe addiction (dependence) to opioid drugs. Local Coverage Determinations (LCDs) do not exist at this time.
