The wider the confidence interval, the less precise is our estimate of the treatment effect. This precision depends on the size of the SE. This is a measure of the spread of the sampling distribution, which in turn depends on the sample size.
What makes an estimate of treatment effect more precise?
All estimates are just that, estimates, and thus they will have some degree of uncertainty about them. The less uncertainty there is about an estimate due to chance variability, the more precise the estimate is said to be. To answer this question look at the confidence interval (or P value) for each estimate of treatment effect.
What is the size of the treatment effect?
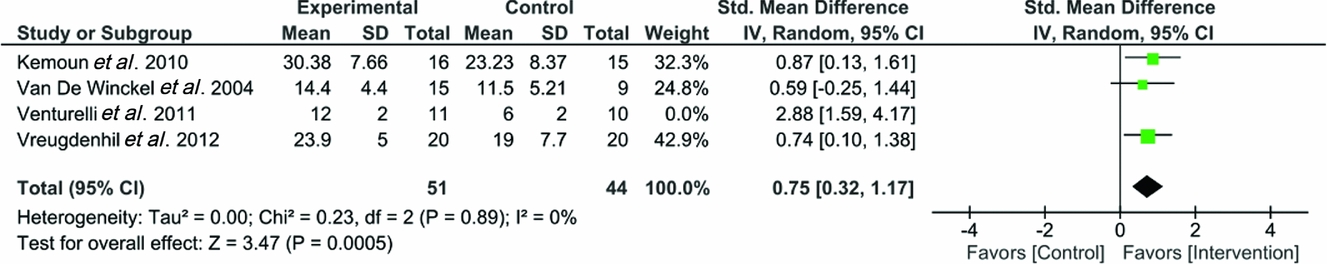
The best estimate of the size of the treatment effect (-1 per cent) has been illustrated as a small square, and the 95 per cent confidence interval about this estimate (-19 to 17 per cent) is shown as a horizontal line. The effect is clearly smaller than the smallest clinically worthwhile effect.
What is the true effect of a treatment?
The true effect of a treatment cannot be known; what we know is an estimate of effect. Confidence intervals are a statistical device to let us know the level of uncertainty around an estimate. The 95% confidence interval (CI) represents the range within which we are 95% certain that the true value of the effect lies.
How to determine whether the effect of treatment is clinically worthwhile?
Modified estimates of treatment effect size can be used to determine if the effect of treatment is likely to be large enough to be “clinically worthwhile”. This approach optimises clinical decision-making by combining unbiased estimates of the size of treatment effect from clinical trials with clinical intuition and patient preferences. How to ...

What was the precision of the estimates of treatment effect?
Recalling that the observed treatment effect is only an estimate of the true effect of the intervention, we would like to have some measure of the uncertainty surrounding the treatment estimate. This precision is usually communicated with a 95% confidence interval (CI).
How do you estimate the treatment effect?
CONTINUOUS MEASURES When a trial uses a continuous measure, such as blood pressure, the treatment effect is often calculated by measuring the difference in mean improvement in blood pressure between groups. In these cases (if the data are normally distributed), a t-test is commonly used.
What is a treatment effect in statistics?
Treatment effects can be estimated using social experiments, regression models, matching estimators, and instrumental variables. A 'treatment effect' is the average causal effect of a binary (0–1) variable on an outcome variable of scientific or policy interest.
How large was the treatment effect meaning?
An estimate of how large the treatment effect is, that is how well the intervention worked in the. experimental group in comparison to the control.
What is an effect estimate?
Effect size estimates provide important information about the impact of a treatment on the outcome of interest or on the association between variables. • Effect size estimates provide a common metric to compare the direction and strength of the relationship between variables across studies.
What is the treatment effect in Anova?
The ANOVA Model. A treatment effect is the difference between the overall, grand mean, and the mean of a cell (treatment level). Error is the difference between a score and a cell (treatment level) mean.
What is treatment effect in clinical trial?
Usually, as with other drug evaluations, the placebo-adjusted treatment effect (i.e., the difference between weight losses with pharmacotherapy and placebo, when given as an adjunct to lifestyle intervention) is provided from data in randomized clinical trials (RCTs).
What is the average treatment effect on the treated?
Average treatment effects on the treated (ATT) and the untreated (ATU) are useful when there is interest in: the evaluation of the effects of treatments or interventions on those who received them, the presence of treatment heterogeneity, or the projection of potential outcomes in a target (sub-) population.
What is the type of error where we wrongly accept the null hypothesis of no treatment effect?
Similarly, even if we can not exclude chance as the explanation of the result from our study, it does not necessarily mean that the treatment is ineffective. This type of error—a false negative result—where we wrongly accept the null hypothesis of no treatment effect is called a type II error .
What is the SE of a study?
The SE is regarded as the unit that measures the likelihood that the result is not because of chance.
How many times should a 6 come up in unbiased dice?
We know that, on average, each of the 6 numbers should come up an equal number of times in unbiased dice. However, when your friend throws 2 or even 3 sixes in a row, you are unlikely (depending on the friend) to infer that the dice are loaded (biased) or that he or she is cheating.
Is a treatment effect statistically significant?
However, just because a test shows a treatment effect to be statistically significant, it does not mean that the result is clinically important. For example, if a study is very large (and therefore has a small standard error), it is easier to find small and clinically unimportant treatment effects to be statistically significant. A large randomised controlled trial compared rehospitalisations in patients receiving a new heart drug with patients receiving usual care. A 1% reduction in rehospitalisation was reported in the treatment group (49% rehospitalisations v 50% in the usual care group). This was highly statistically significant (p<0.0001) mainly because this is a large trial. However, it is unlikely that clinical practice would be changed on the basis of such a small reduction in hospitalisation.
Why are trials stopped early?
At times, trials are stopped early and reported because of positive, large treatment effects . However, early termination may introduce bias secondary to chance deviations from the “true effect” of treatment which would decrease if the trial was continued to completion.[15] .
What should urologists consider when making treatment decisions?
Finally, urologists should consider all patient-important outcomes as well as the balance of potential benefits, harms, and costs, and patient values and preferences when making treatment decisions. Conclusion:
Why is prognostic balance less certain?
At study's completion, the question of prognostic balance is less certain because of a relatively high rate of loss to follow-up.
Why is follow up important at the end of a trial?
In order to assure that both experimental and control groups are balanced at the end of a trial, complete follow-up information on each patient enrolled is important. Unfortunately, this is rarely the case at the close of a trial. Therefore, it is important to understand to what extent follow-up was incomplete.
Do RCTs have meta-analysis?
Ideally, a systematic review and meta-analysis of several randomized controlled trials (RCTs) will exist to guide treatment decisions. However, RCTs comprise a very small proportion of the urologic literature,[3] which inhibits meta-analysis.
Should urology trials be terminated early?
For this reason, critical readers of the urology literature should interpret trials terminated early with caution. In the case of the REDUCE trial, it appears that the trial went to completion, so this is not a concern in terms of the validity of the trial.
How can a randomised trial help in clinical decision making?
Properly conducted randomised trials can aid clinical decision-making by providing unbiased estimates of the average size of treatment effects. This paper, the first of two, discusses how readers of clinical trials can extract simple estimates of treatment effect size from trial reports when trial outcomes are measured on a continuous scale.
What is the purpose of clinical trials?
They must, in addition, ascertain how big the treatment effect is. Good clinical trials provide unbiased estimates of the size of a treatment's effects. Such estimates can be used to determine if a treatment has a big enough effect to be clinically worthwhile.
What does high p mean in a trial?
High p values are properly interpreted as a lack of evidence of a treatment effect. A consequence of this tortuous logic is to distract readers from the most important piece of information that a trial can provide, that is, information about the magnitude of the treatment's effects.
Do trials report change in outcome variables?
Some trials will, instead, report the change in outcome variables over the treatment period. In such trials, the measure of the size of the treatment's effect is still the difference of the means (this time of the difference of the mean change) in treatment and control groups.
Can clinical trials tell us what the effect of a treatment will be for a particular patient?
Thus, while clinical trials cannot tell us what the effect of a treatment will be for a particular patient, they can tell us what the most likely effect will be. The same limitation applies to all sources of information about treatment effects - this is not a limitation unique to clinical trials.
Can clinical trials be used to estimate the average effect of a treatment?
Clinical trials can provide an estimate of the average effects of treatment. Fortunately, knowing about the average effects of treatment is usually the same as knowing about the most probable effects of treatment - usually they are, in fact, the same thing.
Do clinical trials provide information about the cost of treatment?
Clinical trials often provide information about the size of treatment effects, but they rarely provide information about the costs of treatment.
ATT and ATU
The former is the average treatment effect for the individuals which are treated, and for which a particular explanatory variable describing their treatment X i \color {#7A28CB}X_i X i is equal to 1 1 1.
Simple Difference In Mean Outcomes
Let’s recall what values I can calculate given the outcomes I observe when inferring the causal effect of images in email alerts on my email subscribers.
Extension To Regression
Often times, the SDO estimation of an ATE can be calculated with a linear regression, which models a linear relationship between explanatory variables and outcome variables. Consider the following switching equation presented in my previous post:
How Can We Deal With Bias In An ATE Estimation?
Ok, so we understand the ways in which the simple difference in mean outcomes for ATE estimation can be significantly biased away from the true ATE.