Is Regeneron better than remdesivir?
Oct 12, 2021 · A tweet with more than 11,000 likes at the time of writing stated that Regeneron's monoclonal antibody treatment for COVID-19, known as REGEN-COV, costs the U.S. government $2,100 per dose. Trial...
Who pays for Regeneron treatment?
Aug 18, 2021 · According to the New York Times, the dosages of the treatment are available for free, but some patients may pay a fee to have the drug given. It is up to the health insurers and health care providers. In January, the federal government ordered 1.5 million doses of the drug from Regeneron and shipped nearly 300,000 of them.
How expensive is Regeneron?
Aug 25, 2021 · How much does monoclonal antibody treatment cost? The cost of Regeneron’s two-drug cocktail is $1,250 per infusion, according to Kaiser Health News. The federal government currently covers this....
Does Medicare cover Regeneron?
Aug 29, 2021 · In November 2020, the FDA granted emergency use authorization for providers to use the antibody treatment for their clients. In August 2021, the FDA expanded Regeneron’s Monoclonal Antibody Infusion to include post-exposure prophylaxis. This means those that have been exposed to COVID-19 and are at high-risk of becoming positive and ...
How many types of monoclonal antibody COVID-19 treatments are there in the US?
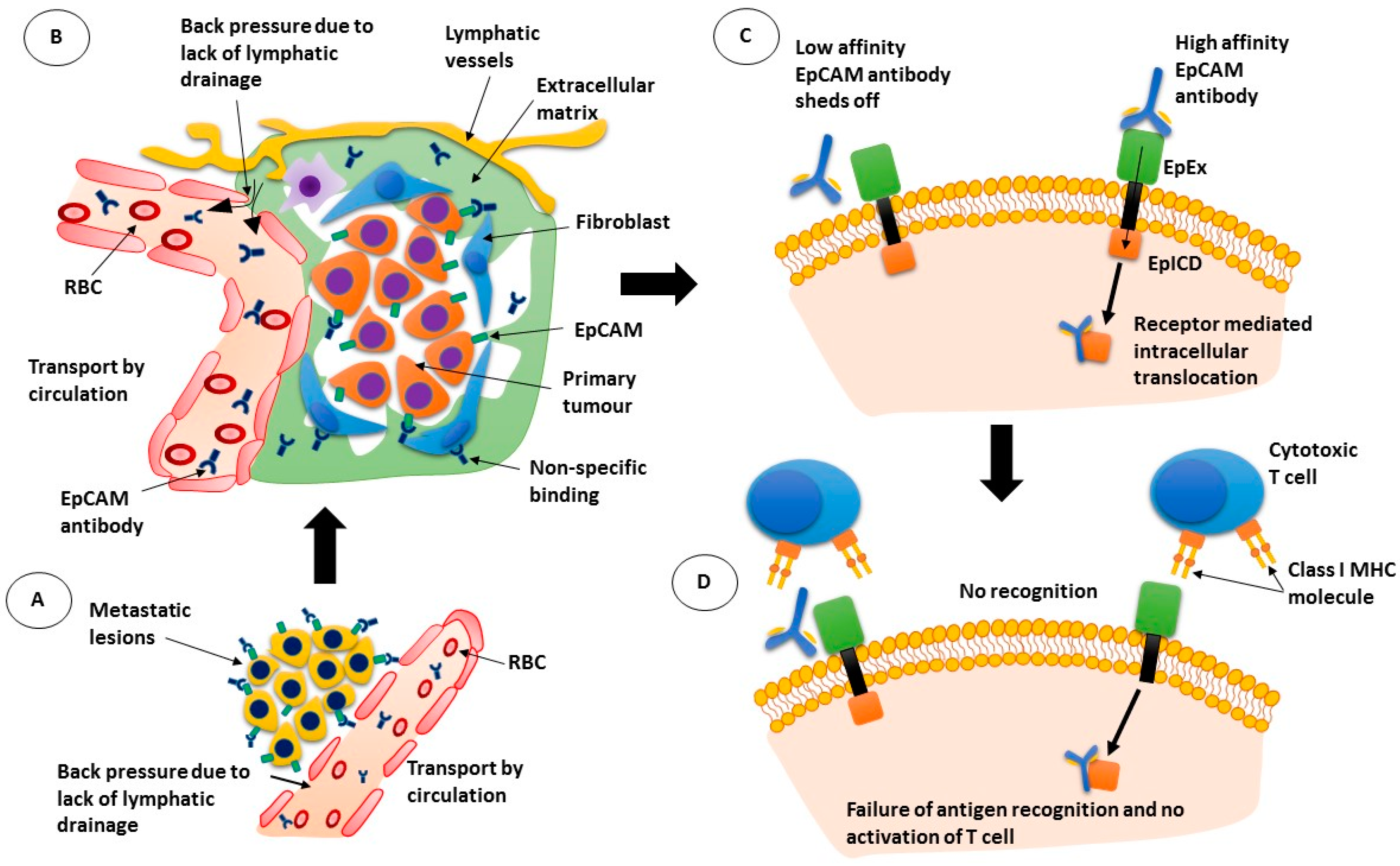
In the United States, there are three anti-SARS-CoV-2 monoclonal antibody treatments with FDA Emergency Use Authorization (EUA) for the treatment of COVID-19: bamlanivimab plus etesevimab, casirivimab plus imdevimab,, and sotrovimab.
What is a monoclonal antibody for COVID-19?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells. Monoclonal antibodies for COVID-19 may block the virus that causes COVID-19 from attaching to human cells, making it more difficult for the virus to reproduce and cause harm. Monoclonal antibodies may also neutralize a virus.Mar 31, 2022
What is the difference between monoclonal antibodies and the COVID-19 vaccine?
COVID-19 vaccines help stimulate and prepare a person's immune system to respond if they are exposed to the virus. However, monoclonal antibodies boost the immune system only after a person is already sick, speeding up their immune response to prevent COVID-19 from getting worse.Nov 8, 2021
How long do COVID-19 antibodies last?
At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.Jan 31, 2022
What is a monoclonal antibody?
Monoclonal antibodies are laboratory-produced molecules that act as substitute antibodies that can restore, enhance or mimic the immune system's attack on cells.Mar 31, 2022
Can I get the COVID-19 vaccine if I was treated with monoclonal antibodies or convalescent plasma?
If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
Should you still get the COVID-19 vaccine if you were treated with monoclonal antibodies?
If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, there is no need to delay getting a COVID-19 vaccine.Feb 17, 2022
Do I need the COVID-19 vaccine if I still have antibodies?
Yes, the COVID-19 vaccines are recommended, even if you had COVID-19.Nov 23, 2021
Can I get COVID-19 again after having the vaccine?
Getting COVID-19 after you've been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you.Nov 9, 2021
Can you get COVID-19 if you already had it and have antibodies?
It is important to remember that some people with antibodies to SARS-CoV-2 may become infected after vaccination (vaccine breakthrough infection) or after recovering from a past infection (reinfected).Nov 10, 2021
How long do antibodies last in people who have mild COVID-19 cases?
A UCLA study shows that in people with mild cases of COVID-19, antibodies against SARS-CoV-2 — the virus that causes the disease — drop sharply over the first three months after infection, decreasing by roughly half every 36 days. If sustained at that rate, the antibodies would disappear within about a year.
How long does it take for antibodies to develop after exposure to COVID-19?
It can take days to weeks after an infection for your body to make antibodies.Feb 24, 2022
Monoclonal Antibody Infusion for COVID-19 Available at Regen IV Wellness
Here at Regen IV Wellness, we are committed to doing our part in helping our clients and our community in battling COVID-19 and its debilitating symptoms. One of the ways that we have seen significant improvement in our client’s that have been diagnosed with COVID-19 is the Regeneron Monoclonal Antibody Infusion.
How does Regeneron Monoclonal Antibody Infusion work?
The moment a person becomes infected with COVID-19, our bodies begin to produce antibodies to fight back the infection. Unfortunately, your body’s response and production of these antibodies takes time to produce enough to beat back the infection.
Can most patients tolerate antibody treatment?
Monoclonal antibodies are already used to fight many cancers and autoimmune diseases and are generally well-tolerated by patients. Most doctors believe that Regeneron’s cocktail and its impact on clients is similar to other antibody treatments that have been used for many years.
Has the FDA weighed-in on Regeneron?
In November 2020, the FDA granted emergency use authorization for providers to use the antibody treatment for their clients. In August 2021, the FDA expanded Regeneron’s Monoclonal Antibody Infusion to include post-exposure prophylaxis.
What exactly is in a monoclonal antibody treatment and how do they work?
In the United States, there are three monoclonal antibody treatments with FDA emergency use authorization for the treatment of COVID-19: bamlanivimab plus etesevimab, developed by Eli Lilly; casirivimab plus imdevimab, made by Regeneron Pharmaceuticals; and sotrovimab, which is manufactured by GlaxoSmithKline.
Who is eligible for monoclonal antibody treatment?
If you believe you are at high risk for progression of severe COVID-19, including hospitalization or death, you may be eligible for the the COVID-19 antibody cocktails.
How effective is it?
Ginde said it can be a life-saving treatment when administered in time. Numerous trials have shown that the treatment can be effective at reducing the risk of hospitalization and death for people at risk of severe COVID.
When do I need to get the treatment in order for it to work?
The monoclonal antibody treatments are meant for mild to moderate COVID cases in adults and children over 12 to prevent the progression of severe COVID.
How can I get a monoclonal antibody treatment for COVID-19?
The ease of access varies state by state, as the Department of Health and Human Services determines how much of the national supply gets distributed on a weekly basis. Then, different state and territorial health departments decide which areas receive it and how much.
Are there side effects?
It’s rare but possible to have side effects. At least 1% of subjects receiving Regeneron’s antibody cocktail in a Phase 3 trial got skin redness and itchiness at the injection site, according to the FDA.
How much does it cost?
The federal government is covering the cost of the monoclonal antibody therapies, so it is free to get, but there might be an administration cost billed to your insurance if you have one.