How big of a deal is CRISPR?
4 rows · Dec 11, 2021 · How much does CRISPR treatment cost? The cost of treatment is a concern. Treating sickle ...
How much does CRISPR cost?
According to a base-case analysis conducted by the Institute for Clinical and Economic Review (ICER), Zolgensma’s subjective value to their patients has been estimated to be around $900,000 per treatment. Still, nothing is stopping Novartis from inflating the price.
How good is CRISPR?
How Much Will Crispr Cost?The cost of treatment is a concern Treating sickle cell disease with CRISPR therapy, Doudna said, costs about $2 million a patient.Jun 30, 2021Who will pay for CRISPR?Boston-based Vertex will pay CRISPR $900 million upfront with a potential for $200 million in milestone pa
Is CRISPR helping with cancer treatment?
What makes CRISPR so revolutionary is that it’s so precise: The Cas9 enzyme mostly goes wherever you tell it to. And it’s incredibly cheap and easy: In the past, it might have cost thousands of dollars and weeks or months of fiddling to alter a gene. Now it might cost just $75 and only take a few hours.

How much does CRISPR cost per patient?
This is a key point. Take the example of sickle cell disease: the first wave of clinical trials on CRISPR-based therapies is looking quite promising in terms of safety and efficacy, but right now the procedure costs in excess of $2 million per patient.Oct 21, 2021
Do you have to pay to use CRISPR?
For academic and non-profit research use, no written license is necessary. For these communities we make CRISPR tools, knowledge, methods and other IP for genome-editing freely available for research.
Is CRISPR cheaper than other methods?
The CRISPR-Cas9 system has generated a lot of excitement in the scientific community because it is faster, cheaper, more accurate, and more efficient than other genome editing methods.Mar 22, 2022
How much does gene therapy cost?
At an upfront price of $2.125 million, the one-time gene therapy onasemnogene abeparvovec for spinal muscular atrophy, a rare neuromuscular disorder that is usually fatal by 2 years of age if untreated, has been called the "most expensive drug ever." This flawed characterization raises important methodological and ...
Will CRISPR be covered by insurance?
That means insurance companies likely won't pay for treatments using CRISPR until there's enough data available that demonstrates its effectiveness. Generally though, he said, they will pay for therapies approved by the FDA.Jun 10, 2018
Who will pay for CRISPR?
The companies announced Tuesday that Vertex will pay CRISPR Therapeutics $900 million up front to change terms of the deal that had both companies split the costs and potential profits from sales of CTX001, a therapy currently in clinical development as a cure for sickle cell disease and transfusion-dependent beta ...Apr 20, 2021
Is CRISPR cheap or expensive?
How much does CRISPR treatment cost? Treating sickle cell disease with CRISPR therapy, Doudna said, costs about $2 million a patient.Dec 11, 2021
Why is CRISPR this affordable?
What makes CRISPR so revolutionary is that it's so precise: The Cas9 enzyme mostly goes wherever you tell it to. And it's incredibly cheap and easy: In the past, it might have cost thousands of dollars and weeks or months of fiddling to alter a gene. Now it might cost just $75 and only take a few hours.Dec 27, 2018
What is CRISPR used for today?
Using the CRISPR system, researchers can precisely edit any target DNA locus - a feat that was not achievable using other gene editing tools. The possibility to edit a disease mutation to correct genetic errors creates opportunities for treating conditions that have long eluded the medical research community.Mar 23, 2021
How long will gene therapy last?
The new guidelines suggest that studies using integrating vectors and genome-editing products follow patients for at least 15 years, while for adeno-associated viral vectors, a minimum 5-year follow-up period is recommended.Apr 15, 2021
Is gene therapy a one-time treatment?
Gene therapy is a new generation of medicine where a functioning gene is delivered to a targeted tissue in the body to produce a missing or nonfunctioning protein. By using genes as medicine, the underlying cause of a disease can be targeted at the cellular level, potentially with just one treatment.
Is gene therapy permanent or temporary?
Gene therapy offers the possibility of a permanent cure for any of the more than 10,000 human diseases caused by a defect in a single gene.
How much does Zolgensma cost?
But here’s the catch: Novartis has priced the gene therapy at $2 million per treatment.
How rare is SMA?
SMA is a relatively rare disease . Only an estimated 700 patients are eligible to receive the treatment. With expensive R&D costs and clinical trials, pharmaceutical companies still intend to recoup their losses. To compensate for the tiny customer base, the pharmaceutical company made the price sky high.
Who is Alison Irvine?
Alison Irvine is a science writer at Memorial Sloan Kettering Cancer Center. She has a special interest in the ethical implications of emerging biotechnologies, and has written about biodesign and bioethics for venues including Popular Science Magazine and The Center for Genomics & Society.
Who is Ross Wilson?
Ross Wilson, Innovative Genomics Institute. Ross Wilson, Ph.D., is a Principal Investigator at the IGI. His lab works with enzymes such as Cas9 to test their efficiency for use in genome editing therapies.
How does CRISPR work?
With other versions of CRISPR, scientists can manipulate genes in more precise ways such as adding a new segment of DNA or editing single DNA letters . Scientists have also used CRISPR to detect specific targets, such as DNA from cancer-causing viruses and RNA from cancer cells.
What is CRISPR 2020?
July 27, 2020 , by NCI Staff. CRISPR is a highly precise gene editing tool that is changing cancer research and treatment. Credit: Ernesto del Aguila III, National Human Genome Research Institute. Ever since scientists realized that changes in DNA cause cancer, they have been searching for an easy way to correct those changes by manipulating DNA.
What is the CRISPR enzyme?
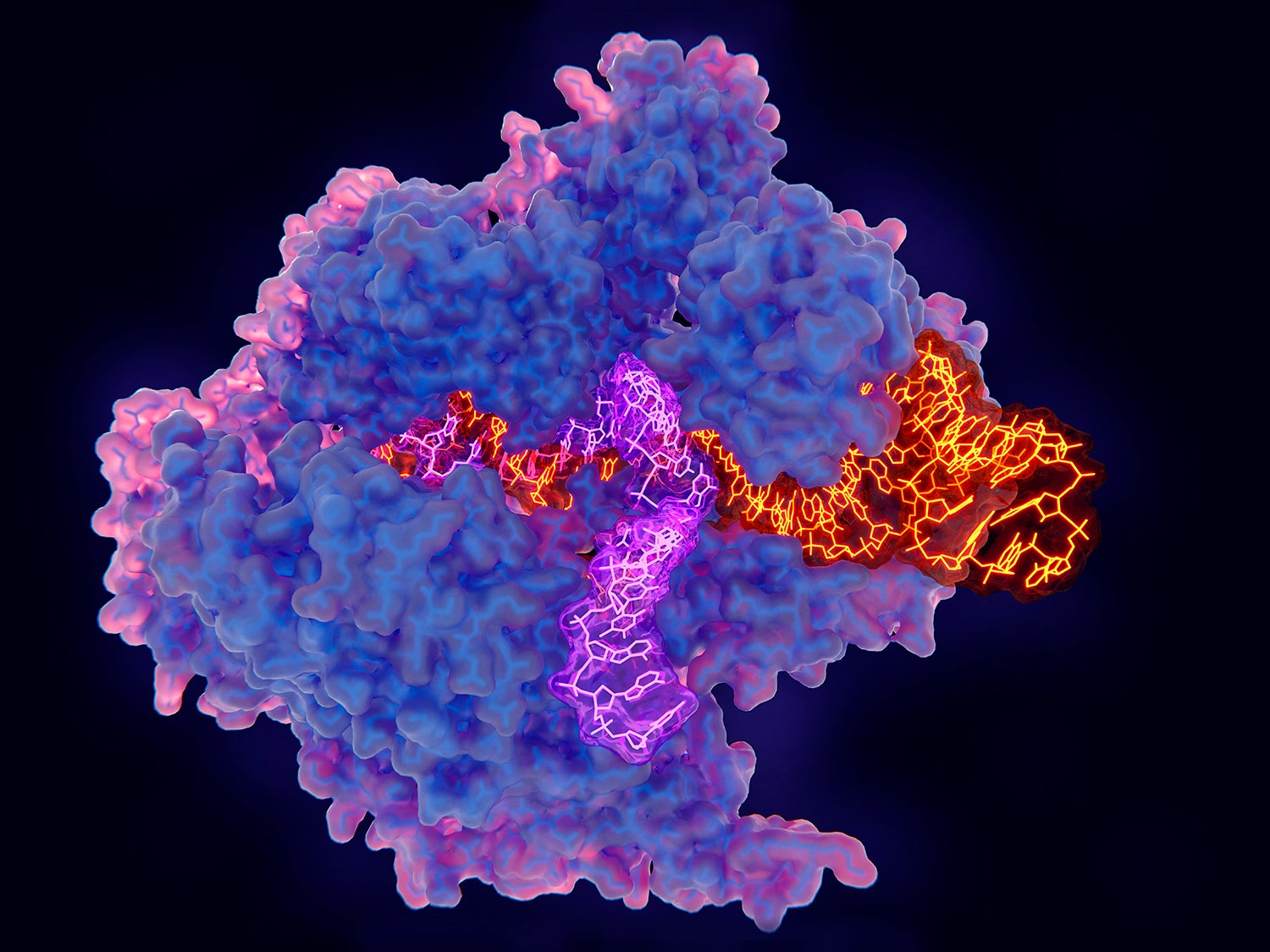
CRISPR consists of a guide RNA (RNA-targeting device, purple) and the Cas enzyme (blue). When the guide RNA matches up with the target DNA (orange), Cas cuts the DNA. A new segment of DNA (green) can then be added. Credit: National Institute of General Medical Sciences, National Institutes of Health.
What was the first trial of CRISPR?
The first trial of CRISPR for patients with cancer tested T cells that were modified to better "see" and kill cancer. CRISPR was used to remove three genes: two that can interfere with the NY-ESO-1 receptor and another that limits the cells’ cancer-killing abilities.
How long does it take to make a mouse model?
And gene editing with CRISPR is a lot faster. With older methods, “it usually [took] a year or two to generate a genetically engineered mouse model, if you’re lucky,” said Dr. Li. But now with CRISPR, a scientist can create a complex mouse model within a few months, he said.
Is CRISPR good for cancer?
There’s also hope that it will have a place in treating cancer, too. But CRISPR isn’t perfect, and its downsides have made many scientists cautious about its use in people. A major pitfall is that CRISPR sometimes cuts DNA outside of the target gene—what’s known as “off-target” editing.
How much does Kymriah cost?
Kymriah, the recently-approved treatment that delivers an engineered immune system protein in gene therapy wrapping, is a one-time treatment for a form of leukemia, costing $475,000. Yescarta, for a different blood cancer, is similarly priced. The seven-figure cap may come from experience with Glybera, the first gene therapy approved in Europe.
How much does Strimvelis cost?
The second gene therapy approved in Europe, Strimvelis, to treat an inherited immune deficiency, costs $665,000.
Who is Ricki Lewis?
Ricki Lewis has a PhD in genetics and is a genetics counselor, science writer and author of Human Genetics: The Basics. Follow her at her website or Twitter @rickilewis. The GLP featured this article to reflect the diversity of news, opinion and analysis. The viewpoint is the author’s own.
When was gene therapy first used?
That’s a matter of intense focus right now. The first clinical trial for a gene therapy was way back in 1990. But over the past year or two, treatments are finally nearing the end of the regulatory pathway.
When will Luxturna be available?
That’s how Luxturna, the blindness treatment, will be rolled out in 2018.
Who's loving you Christian Guardino?
Christian Guardino, then 16, already demonstrated the gene therapy success when he sang the Jackson 5’s “Who’s Lovin’ You” on America’s Got Talent last June. Before gene therapy, he’d been unable to recognize faces, walk the hallways of his school, or stay outside safely once the sun went down.
When did the Biologics Price Competition and Innovation Act start?
In 2009, the Biologics Price Competition and Innovation Act created a pathway for approving generic biologics, also known as biosimilars. It may apply to CRISPR-based biosimilars, but generic gene-editing — and thus competition to drive down prices — is unlikely to appear for decades.
Can CRISPR be used to alter genes?
CRISPR will allow us to alter our existing genes. But it often relies on using viruses to shuttle the molecular gene-editing systems into our cells, and can be as expensive as other gene therapies. Editas Medicine plans to use CRISPR-Cas9 to treat various diseases, including Leber congenital amaurosis.
What is the concern with CRISPR?
A major concern for implementing CRISPR/Cas9 for gene therapy is the relatively high frequency of off-target effects (OTEs), which have been observed at a frequency of ≥50% ( 31 ). Current attempts at addressing this concern include engineered Cas9 variants that exhibit reduced OTE and optimizing guide designs. One strategy that minimizes OTEs utilizes Cas9 nickase (Cas9n), a variant that induces single-stranded breaks (SSBs), in combination with an sgRNA pair targeting both strands of the DNA at the intended location to produce the DSB ( 32 ). Researchers have also developed Cas9 variants that are specifically engineered to reduce OTEs while maintaining editing efficacy ( Table 1 ). SpCas9-HF1 is one of these high-fidelity variants that exploits the “excess-energy” model which proposes that there is an excess affinity between Cas9 and target DNA which may be enabling OTEs. By introducing mutations to 4 residues involved in direct hydrogen bonding between Cas9 and the phosphate backbone of the target DNA, SpCas9-HF1 has been shown to possess no detectable off-target activity in comparison to wildtype SpCas9 ( 35 ). Other Cas9 variants that have been developed include evoCas9 and HiFiCas9, both of which contain altered amino acid residues in the Rec3 domain which is involved in nucleotide recognition. Desensitizing the Rec3 domain increases the dependence on specificity for the DNA:RNA heteroduplex to induce DSBs, thereby reducing OTEs while maintaining editing efficacy ( 38, 39 ). One of the more recent developments is the Cas9_R63A/Q768A variant, in which the R63A mutation destabilizes R-loop formation in the presence of mismatches and Q768A mutation increases sensitivity to PAM-distal mismatches ( 49 ). Despite the different strategies, the rational for generating many Cas9 variants with reduced OTEs has been to ultimately reduce general Cas9 and DNA interactions and give a stronger role for the DNA:RNA heteroduplex in facilitating the edits.
What is CRISPR in gene therapy?
The discovery of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) proteins has expanded the applications of genetic research in thousands of laboratories across the globe and is redefining our approach to gene therapy. Traditional gene therapy has raised some concerns, as its reliance on viral vector delivery of therapeutic transgenes can cause both insertional oncogenesis and immunogenic toxicity. While viral vectors remain a key delivery vehicle, CRISPR technology provides a relatively simple and efficient alternative for site-specific gene editing, obliviating some concerns raised by traditional gene therapy. Although it has apparent advantages, CRISPR/Cas9 brings its own set of limitations which must be addressed for safe and efficient clinical translation. This review focuses on the evolution of gene therapy and the role of CRISPR in shifting the gene therapy paradigm. We review the emerging data of recent gene therapy trials and consider the best strategy to move forward with this powerful but still relatively new technology.
How did gene therapy begin?
The birth of gene therapy as a therapeutic avenue began with the repurposing of viruses for transgene delivery to patients with genetic diseases. Gene therapy enjoyed an initial phase of excitement, until the recognition of immediate and delayed adverse effects resulted in death and caused a major setback. More recently, the discovery and development of CRISPR/Cas9 has re-opened a door for gene therapy and changed the way scientists can approach a genetic aberration—by fixing a non-functional gene rather than replacing it entirely, or by disrupting an aberrant pathogenic gene. CRISPR/Cas9 provides extensive opportunities for programmable gene editing and can become a powerful asset for modern medicine. However, lessons learned from traditional gene therapy should prompt greater caution in moving forward with CRISPR systems to avoid adverse events and setbacks to the development of what may be a unique clinically beneficial technology. A failure to take these lessons into account may provoke further backlash against CRISPR/Cas9 development and slow down progression toward attaining potentially curative gene editing technologies.
Who is CR consulted with?
CR has consulted regarding oncology drug development with AbbVie, Amgen, Ascentage, Astra Zeneca , Celgene, Daiichi Sankyo, Genentech/Roche, Ipsen, Loxo, and Pharmar, and is on the scientific advisory boards of Harpoon Therapeutics and Bridge Medicines. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
What is the CRISPR locus?
The bacterial CRISPR locus was first described by Francisco Mojica ( 23) and later identified as a key element in the adaptive immune system in prokaryotes ( 24 ). The locus consists of snippets of viral or plasmid DNA that previously infected the microbe (later termed “spacers”), which were found between an array of short palindromic repeat sequences. Later, Alexander Bolotin discovered the Cas9 protein in Streptococcus thermophilus, which unlike other known Cas genes, Cas9 was a large gene that encoded for a single-effector protein with nuclease activity ( 25 ). They further noted a common sequence in the target DNA adjacent to the spacer, later known as the protospacer adjacent motif (PAM)—the sequence needed for Cas9 to recognize and bind its target DNA ( 25 ). Later studies reported that spacers were transcribed to CRISPR RNAs (crRNAs) that guide the Cas proteins to the target site of DNA ( 26 ). Following studies discovered the trans-activating CRISPR RNA (tracrRNA), which forms a duplex with crRNA that together guide Cas9 to its target DNA ( 27 ). The potential use of this system was simplified by introducing a synthetic combined crRNA and tracrRNA construct called a single-guide RNA (sgRNA) ( 28 ). This was followed by studies demonstrating successful genome editing by CRISPR/Cas9 in mammalian cells, thereby opening the possibility of implementing CRISPR/Cas9 in gene therapy ( 29) ( Figure 1 ).
Does CRISPR cause apoptosis?
CRISPR- induced DSBs often trigger apoptosis rather than the intended gene edit ( 68 ). Further safety concerns were revealed when using this tool in human pluripotent stem cells (hPSCs) which demonstrated that p53 activation in response to the toxic DSBs introduced by CRISPR often triggers subsequent apoptosis ( 69 ). Thus, successful CRISPR edits are more likely to occur in p53 suppressed cells, resulting in a bias toward selection for oncogenic cell survival ( 70 ). In addition, large deletions spanning kilobases and complex rearrangements as unintended consequences of on-target activity have been reported in several instances ( 71, 72 ), highlighting a major safety issue for clinical applications of DSB-inducing CRISPR therapy. Other variations of Cas9, such as catalytically inactive endonuclease dead Cas9 (dCas9) in which the nuclease domains are deactivated, may provide therapeutic utility while mitigating the risks of DSBs ( 73 ). dCas9 can transiently manipulate expression of specific genes without introducing DSBs through fusion of transcriptional activating or repressing domains or proteins to the DNA-binding effector ( 74 ). Other variants such as Cas9n can also be considered, which induces SSBs rather than DSBs. Further modifications of these Cas9 variants has led to the development of base editors and prime editors, a key innovation for safe therapeutic application of CRISPR technology (see Precision Gene Editing With CRISPR section).
What is the purpose of CRISPR/CAS9?
CRISPR/Cas9 is a simple two-component system used for effective targeted gene editing. The first component is the single-effector Cas9 protein, which contains the endonuclease domains RuvC and HNH. RuvC cleaves the DNA strand non-complementary to the spacer sequence and HNH cleaves the complementary strand. Together, these domains generate double-stranded breaks (DSBs) in the target DNA. The second component of effective targeted gene editing is a single guide RNA (sgRNA) carrying a scaffold sequence which enables its anchoring to Cas9 and a 20 base pair spacer sequence complementary to the target gene and adjacent to the PAM sequence. This sgRNA guides the CRISPR/Cas9 complex to its intended genomic location. The editing system then relies on either of two endogenous DNA repair pathways: non-homologous end-joining (NHEJ) or homology-directed repair (HDR) ( Figure 2 ). NHEJ occurs much more frequently in most cell types and involves random insertion and deletion of base pairs, or indels, at the cut site. This error-prone mechanism usually results in frameshift mutations, often creating a premature stop codon and/or a non-functional polypeptide. This pathway has been particularly useful in genetic knock-out experiments and functional genomic CRISPR screens, but it can also be useful in the clinic in the context where gene disruption provides a therapeutic opportunity. The other pathway, which is especially appealing to exploit for clinical purposes, is the error-free HDR pathway. This pathway involves using the homologous region of the unedited DNA strand as a template to correct the damaged DNA, resulting in error-free repair. Experimentally, this pathway can be exploited by providing an exogenous donor template with the CRISPR/Cas9 machinery to facilitate the desired edit into the genome ( 30 ).
