Four mg in 30 mL to 50 mL 0.9% intrapleural sodium chloride injection via chest tube and left to dwell for 1 hour as a single dose or as multiple doses 24 hours apart 0.1 mg/kg/hr (maximum of 20 mg per 24 hours for up to 96 hours)
Full Answer
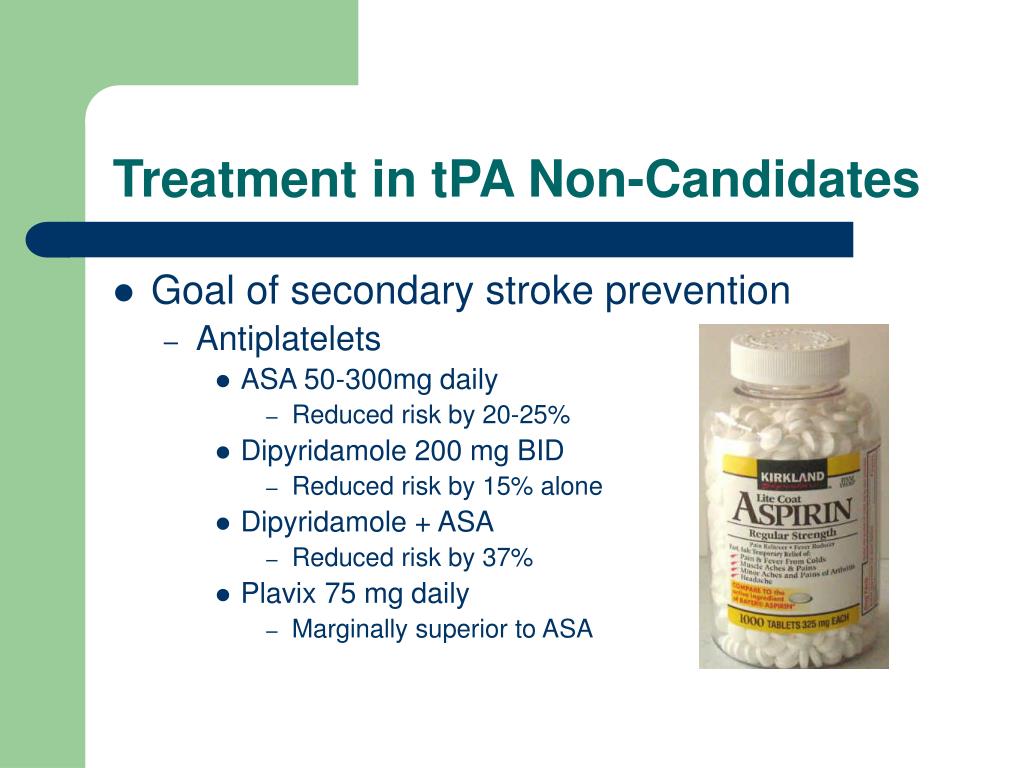
What is the maximum amount of tPA given?
The benefit of tPA depends a lot on time. The sooner the patient receives tPA; the better are the outcomes. Maximum recommended dose is 90mg Patients less than or equal to 100 kg load with 0.09 mg/kg (10% of 0.9 mg/kg dose) as an IV bolus over 1 minute, followed by 0.81 mg/kg (90% of 0.9 mg/kg dose) as a continuous infusion over 60 minutes.
What is tPA used to treat?
Tissue plasminogen activator, or tPA, is the only FDA-approved treatment for ischemic or thrombotic stroke, which is stroke caused by a blood clot interrupting blood flow to a region of the brain. 1 It has also been used in treatment for pulmonary embolism and myocardial infarction.
How much TPA should be taken after a stroke?
100 mg over 2 hours; may be administered as a 10 mg bolus followed by 90 mg over 2 hours Ischemic Stroke:The only blood test that is necessary before tPA usage is the blood glucose level. If the patient is on anticoagulation like coumadin, then only we should do PT, PTT, and INR, etc. The benefit of tPA depends a lot on time.
How many vials of TPA are in a vial?
1 vial of t-PA (alteplase, or Activase 100 mg) or two vials of t-PA (alteplase, or Activase 50 mg each) It is appropriate to mix tPA prior to CT even if it is not used: See below procedure for returning tPA that is mixed but not administered.
What is the dosing for tPA?
The benefit of tPA depends a lot on time. The sooner the patient receives tPA; the better are the outcomes. Patients less than or equal to 100 kg load with 0.09 mg/kg (10% of 0.9 mg/kg dose) as an IV bolus over 1 minute, followed by 0.81 mg/kg (90% of 0.9 mg/kg dose) as a continuous infusion over 60 minutes.
How much tPA is given for a stroke?
The recommended dose of IV-tPA according to the National Institute of Neurological Disorders and Stroke (NINDS) trial was 0.9 mg/kg (maximum 90 mg).
How do you calculate tPA infusion rate?
Calculate dose: (Enter Body weight in kg OR in lb)in Kg: Kg or.in lb: ( lb / 2.2 )Total TPA dose is: weight in kg x 0.9 mg/kg = Total dose mg.Give 10 percent as bolus (10% of total dose as bolus)Infuse remaining dose over 60 minutes.
What size vial is used for tPA administration for the ischemic stroke patient?
50 mg vials - administer using either a polyvinyl chloride bag or glass vial and infusion set. 100 mg vials - remove from the vial any quantity of drug in excess of that specified for patient treatment [see Acute Ischemic Stroke and Acute Myocardial Infarction].
How do you mix tPA for stroke?
1:504:34tPA (Alteplase) Mixing Video - YouTubeYouTubeStart of suggested clipEnd of suggested clipDevice into the vial containing activase a process that requires approximately two minutes aboutMoreDevice into the vial containing activase a process that requires approximately two minutes about half a milliliter of sterile water may remain in the upper.
Why is tPA given within 3 hours?
If a patient arrives at the emergency room within three hours of experiencing stroke symptoms, doctors can administer a potent clot-busting medication and often save critical brain tissue.
What is tPA bolus?
Intravenous tissue plasminogen activator (tPA) (0.9 mg/kg, maximum 90 mg) with a bolus of 10% of the total dose given within 1–2 mins is the standard therapy for patients receiving thrombolytic therapy. Low-dose (0.6 mg/kg) tPA is also approved for thrombolytic therapy for ischemic stroke patients.
How is thrombolytic therapy administered?
The “clot-busting” drug will be delivered through a peripheral intravenous (IV) line, usually through a visible vein in your arm. Performed at your bedside in an intensive care unit while your heart and lung functions are monitored. The drug circulates within the blood stream until it reaches the clot.
When is tPA administered?
When administered quickly after stroke onset (within three hours, as approved by the FDA), tPA helps to restore blood flow to brain regions affected by a stroke, thereby limiting the risk of damage and functional impairment.
How do you give a tPA to a PICC line?
Doses of 1 to 2 mg injected into the lumen(s) of central venous catheters or PICC lines, allowed to dwell for 15 minutes to 4 hours, then removed by aspiration, have been reported effective in establishing patency. Instill 2 mg/2 ml into the dysfunctional catheter for 2 hours.
How do you administer a tPA in a chest tube?
The protocol is: 6 mg of alteplase in 50 mL of normal saline instilled via a pleural chest tube. The chest tube is clamped for 4 hours (dwell time); then, unclamped and allowed to drain. One dose was given per 24 hour period, for a total of three doses.
How many types of tPA are there?
There are two forms of t-PA, single-chain t-PA (sct-PA) and two-chain t-PA (tct-PA). The single chain molecule is the native form of t-PA secreted from endothelial cells, whereas the two-chain form is the result of the proteolytic activity of plasmin.
How long does it take to use TPA?
Clinical guidelines for the early treatment of stroke published jointly by the American Heart Association and American Stroke Association strongly recommend the use of TPA for eligible patients within three hours of symptom onset. Some of the eligibility criteria involved in the decision to use TPA include ...
How does TPA work?
When TPA is injected into a vein, it quickly travels through the blood to reach the clogged blood vessel, where it works by trying to dissolve the blood clot and to restore blood flow to the brain.
What is tissue plasminogen activator?
Tissue plasminogen activator is a powerful agent that dissolves blood clots. It is injected by intravenous administration (IV) for emergency stroke treatment. A stroke is caused by an interruption in blood flow either due to a blood clot ( ischemic stroke) or a bleed ( hemorrhagic stroke) in the brain. TPA is only used for strokes caused by blood ...
How long after stroke can you get TPA?
Intravenous TPA has to be administered within the first few hours after a stroke begins. The start of a stroke is counted from the time that you first notice stroke symptoms. After this very short window of a few hours after a stroke starts, you cannot receive TPA because it might cause more harm than good at that point.
What is TPA in 2021?
Huma Sheikh, MD. on April 21, 2021. Tissue plasminogen activator, most commonly known as TPA, is a powerful blood thinner used for emergency stroke treatment. Approved 20 years ago for the treatment of stroke, it was initially viewed as both revolutionary and risky. Now, twenty years later, stroke treatment has advanced a lot, ...
Is TPA a blood thinner?
Because TPA is a powerful blood thinner, the main side effect is bleeding. Bleeding is a serious complication that can result in a hemorrhagic stroke, which is often more serious than an ischemic stroke.
Do patients ask for TPA?
Most of the time, patients do not ask for TPA. But emergency medical workers are trained to recognize a stroke and emergency rooms are equipped with the staff and provisions to administer TPA when it is necessary.
When tPA is mixed but not administered or the packaging is damaged, should the reconstituted and unused answer?
When tPA is mixed but not administered or the packaging is damaged, the reconstituted and unused tPA should be returned for pharmacy credit
What to do if T-PA is reconstituted?
If t-PA is reconstituted or the packaging is not intact and the medication was not used, place a patient identification label on any container holding reconstituted drug _ t-PA bottle, syringe or IV bag. (Remove blunt canula or needles from syringes.) Place containers in a plastic bag if necessary to prevent spillage.
How long to administer bolus syringe?
Apply label (“BOLUS DOSE”, t-PA, and dosage) to Bolus syringe. (MDs only administer bolus dose over 1 minute.)
How often should you document neurologic findings?
Document neurologic assessment findings at least hourly or more frequently if neurologic changes occur
Who to hand bolus dose syringe to?
Hand the bolus dose syringe to the Stroke Neurologist and verify again the bolus dose, infusion dose and rate and discard dose
Who can verify bolus dose?
Verify the bolus dose, infusion dose and discard dose with the Stroke Neurologist
What records do stroke neurologists use?
Stroke Neurologist will document administration of bolus dose on ED Medication Administration record including time, dose, route, initials and signature
How long does tPA last?
Treatment with tPA has been effective for people with an ischemic stroke as long as it is received intravenously within up to 4.5 hours of the onset of symptoms. 3 Endovascular treatment to remove the clot or deliver tPA at the site of the clot is considered for up to 24 hours after a stroke.
What conditions would make you ineligible to receive treatment with tPA?
Conditions that would make you ineligible to receive treatment with tPA include: 3 . Hemorrhagic stroke (bleeding in the brain) Brain aneurysm or AVM. Recent surgical procedure. Head injuries. Bleeding or blood clotting disorders. Bleeding ulcers. Pregnancy. Blood-thinning medication.
What is the FDA approved treatment for ischemic stroke?
on February 19, 2021. Tissue plasminogen activator, or tPA, is the only FDA-approved treatment for ischemic or thrombotic stroke, which is stroke caused by a blood clot interrupting blood flow to a region of the brain. 1 . Chris Ryan / Getty Images.
What to do if you have a stroke and received tPA?
Eliminating illegal drug usage. Lowering cholesterol and fat levels. Managing diabetes if you have it. Maintaining a healthy blood pressure. If you or a loved one has had a stroke or has received tPA for treatment of a stroke, expect a recovery that may take time. Stroke Recovery and Rehabilitation.
How to maximize your chances of getting a stroke?
The best way to maximize your chances of receiving the most effective treatment for a stroke is to get to the emergency room as soon as possible. A person who is having a stroke may not notice when they are experiencing symptoms.
What are the side effects of a blood thinner?
It is a powerful blood thinner, and serious side effects may occur, including the following: 5. Hemorrhage (bleeding) affecting the brain: Causes headaches, weakness, confusion, loss of consciousness, seizures. Hemorrhage of the digestive system: Causes blood in the stool or stomach pain.
Is TPA used for stroke?
Chris Ryan / Getty Images. It has also been used in treatment for pulmonary embolism and myocardial infarction. TPA is a blood thinner, and therefore it is not used for hemorrhagic strokes or head trauma.
How long does it take to administer a 0.9 mg IV?
0.9 mg/kg IV; not to exceed 90 mg total dose; administer 10% of the total dose as an initial IV bolus over 1 minute and the remainder infused over 60 minutes
How much AMI should I take?
Recommended total dose for AMI is based on patient weight, not to exceed 100 mg, regardless of the selected administration regimen (accelerated or 3 hr)
How long after stroke can you take alteplase?
Patients who present within 3 hr of stroke symptom onset, should be treated with alteplase unless contraindications exist; longer time window (3-4.5 hr after symptom onset) shown to be safe and efficacious for select individuals; treatment of patients with minor neurological symptoms not recommended.
Should a drug be withdrawn from a catheter?
Should serious bleeding in a critical location (e.g., intracranial, gastrointestinal, retroperitoneal, pericardial) occur, therapy should be stopped and the drug should be withdrawn from the catheter
When to inspect parenteral drug products?
Inspect parenteral drug products for particulate matter and discoloration prior to administration whenever solution and container permit
Is thrombocytopenia a hazard?
Exercise caution with patients who have thrombocytopenia, other hemostatic defects (including those secondary to severe hepatic or renal disease), or any condition for which bleeding constitutes a significant hazard or would be particularly difficult to manage because of its location, or who are at high risk for embolic complications (e.g., venous thrombosis in the region of the catheter)
What is a tPA?
Background. Ischemic stroke is a leading cause of morbidity and mortality worldwide and recombinant human tissue-type Plasminogen Activator (tPA) is the prominent among very few therapeutics used in its treatment.
Why is tPA used in stroke?
The rationale behind the use of tPA in ischemic stroke is that by breaking down the clot, recanalization of the occluded blood vessel occurs. The restoration of blood vessel patency is meaningful, however, only if the brain tissue of the ischemic area is still viable.
What is tPA protein?
tPA is a secreted serine protease consisting of a single polypeptide chain with 3 or 4 glycosylation sites and numerous disulfide bonds in its secondary structure [16, 17]. The action of plasmin cleaves tPA into an N-terminal light chain and a C-terminal heavy chain still held together by disulfide bonds [18]. Two-chain tPA has higher catalytic efficiency compared to the single chain form and is essentially constitutively fully active, contrary to the single chain form which only becomes fully active upon binding to fibrin [19]. The catalytic domain of tPA lies towards its C-terminal end and comprises the light chain of the protease [20, 21]. The protein also contains the following domains: an N-terminal fibronectin type III finger domain, an epidermal growth factor-like domain and two kringle domains [16, 22]. Plasminogen binds to the second tPA kringle domain and fibrin binds to the finger domain and the second kringle domain. Finally, inhibition by PAI 1 is achieved by covalent binding of PAI 1 to the catalytic domain and the formation of a complex [21]. The presence of the additional domains beyond the catalytic one not only allows for regulation of the catalytic function, but also suggests hitherto unknown interactions and potentially functions. Indeed, both fibronectin finger and growth factor domains are known modalities involved in protein-protein interactions.
How does TPA affect vessel tone?
For starters, tPA is thought to affect vessel tone [113, 107]. This has been proposed to induce hemodynamic alterations that ultimately hamper the perfusion of the ischemic area, so that hypoperfusion ensues despite restoration of vessel patency [107]. The effect on the vessel tone is considered a direct one on smooth muscle cells, to which tPA signals via integrin avβ3in a non-proteolytic fashion to induce vasoconstriction. The signaling is terminated by the binding of PAI 1 on to tPA, the internalization on the integrin-tPA-PAI 1 complex via LRP-mediated endocytosis and the dissociation of the tPA-PAI 1 complex from the integrin followed by its breakdown [114].
What are the two agents that cleave fibrinogen?
A different concept lies behind the use of ancrod [70, 71] and batroxobin (defibrase) [72]. These two agents cleave fibrinogen, the precursor of fibrin, and essentially tip the balance of coagulation and fibrinolysis towards the breakdown of clots, since the clot cannot grow in the absence of its substrate. Defibrinogenating agents are also thought to decrease blood viscosity, thus increasing blood flow through not completely occluded blood vessels. Ancrod, derived from pit viper venom is thought to have additional modes of action in preventing clot formation. However, clinical trial data have not supported the use of fibrinogen depleting agents in the treatment of stroke [71].
What drugs are used to prevent platelet activation?
These include traditional antiplatelets, such as aspirin [73, 76] or combinations of tPA with more novel antiplatelets, such as glycoprotein IIb/IIIa inhibitors (abciximab [77, 78] and eptifibatide [79]). Combinations of tPA with glycoprotein IIb/IIa inhibitors have not yet shown any clinical benefit; on the contrary, the AbESTT II trial employing abciximab was stopped prematurely due to excessive incidence of intracerebral hemorrhages. However, clinical trials employing abciximab and eptifibatide are still ongoing [80, 81, 82]. Finally, another combination under study is that of argatroban, a thrombin inhibitor, and tPA [83]. An earlier study did not show increased risk or clinical benefit from the use of argatroban as monotherapy [84]. Similar to ancrod, batroxobin and heparin, antiplatelets and argatroban act as indirect thrombolytics, since they interfere with further clot formation.
Is tPA a thrombolytic agent?
Recombinant human tPA produced in mammalian cell lines was introduced as a thrombolytic agent in selected cases of stroke following the results of the National Institute of Neurological Diseases and Stroke (NINDS) study in 1995 [29]. This study consisted of two independently powered trials that both showed clinical benefit from the use of tPA. A European study conducted around the same time also showed clinical improvement, albeit more modest, and the use of tPA was not recommended, for fear that eligible patients could not be easily identified [30]. A second European and Australian study showed no benefit from tPA [31], a fact which delayed the use of the protease in Europe until 2002 when a license was granted, provided that an observational safety study was conducted to assess the safety profile of tPA in routine clinical practice. The results of this study were recently published and confirmed the positive effect of tPA [32], thus further relieving reservations concerning its use as a thrombolytic in stroke. However, tPA is still used only in a small number of ischemic stroke cases (3-8%, [33]), but this percentage greatly increases in specialized stroke centers [28].