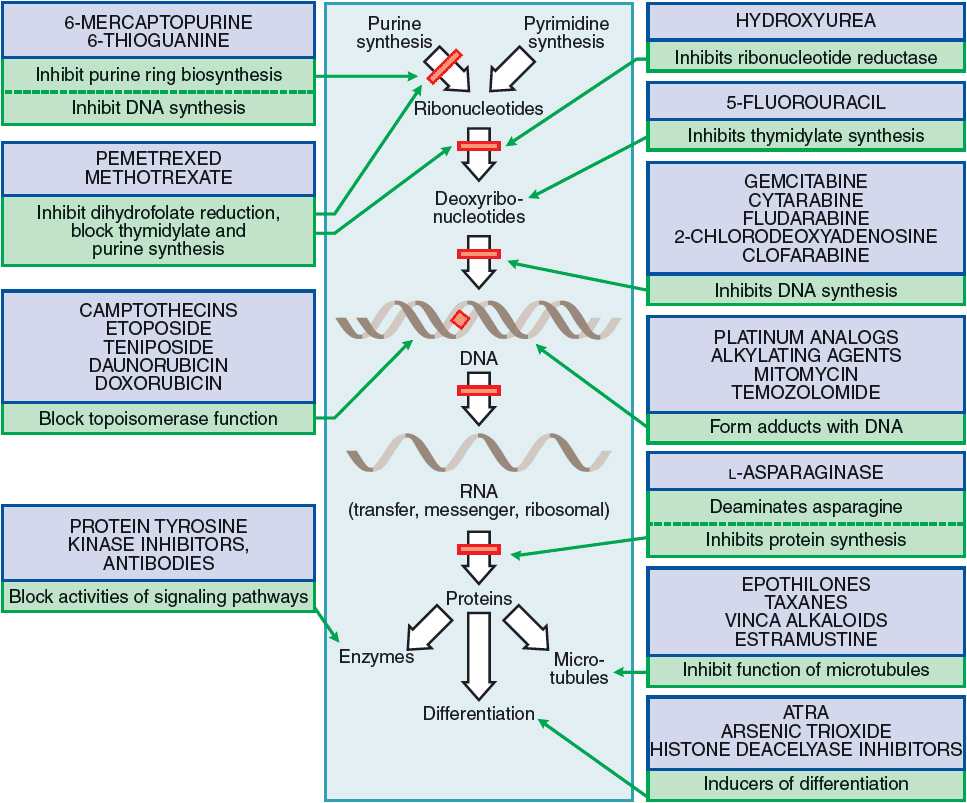
Azaserine is a naturally occurring serine derivative diazo compound with antineoplastic and antibiotic properties deriving from its action as a purinergic antagonist and structural similarity to glutamine. Azazerine acts by competitively inhibiting glutamine amidotransferase, a key enzyme responsible for glutamine metabolism. Mechanism of Action
Full Answer
What is the role of Azaserine in pancreatic cancer?
The first of these uses azaserine as the pancreatic carcinogen in rats. As described above, azaserine induces microscopically detectable AACNs in the exocrine pancreas that can become grossly visible nodules of 1 mm in diameter or larger. The second model uses N-nitrosobis (2-oxopropyl)amine (BOP) administered to the Syrian golden hamster.
What do we know about antitumor activity of Azaserine and Don?
A review of the clinical data on azaserine, DON, and azotomycin reveals that these agents have limited but definite antitumor activity. All three drugs are analogs of L-glutamine and contain a diazo group.
Is Azaserine a pancreatic carcinogen in rats?
The first of these uses azaserine as the pancreatic carcinogen in rats. As described above, azaserine induces microscopically detectable AACNs in the exocrine pancreas that can become grossly visible nodules of 1 mm in diameter or larger.
Does Azaserine cause AACN nodules?
As described above, azaserine induces microscopically detectable AACNs in the exocrine pancreas that can become grossly visible nodules of 1 mm in diameter or larger. The second model uses N-nitrosobis (2-oxopropyl)amine (BOP) administered to the Syrian golden hamster.
Biotransformation
A.J.L. Cooper, M.H. Hanigan, in Comprehensive Toxicology (Third Edition), 2018
Systems Toxicologic Pathology
Matthew A. Wallig, John M. Sullivan, in Haschek and Rousseaux's Handbook of Toxicologic Pathology (Third Edition), 2013
Exocrine Pancreas
Matthew A. Wallig, John M. Sullivan, in Fundamentals of Toxicologic Pathology (Third Edition), 2018
1,3,4-Thiadiazoles
The technique is useful for determining the structure of 1,3,4-thiadiazole with an uncertainty of 0.03 Å for hydrogen atoms and less than 0.003 Å for other atoms 〈68AHC (9)165, p. 199〉.
Exocrine Pancreas
Andrew W. Suttie, ... Melissa Schutten, in Boorman's Pathology of the Rat (Second Edition), 2018
Organ-Specific Toxicologic Pathology
Daniel S. Longnecker, Glenn L. Wilson, in Handbook of Toxicologic Pathology (Second Edition), 2002
Carboxymethylation of DNA Induced by N-Nitroso Compounds and Its Biological Implications
Many carboxymethylated DNA adducts emanating from exposure to NOCs have been identified, and the structures of known carboxymethylated DNA adducts are summarized in Scheme 3. In 1982, Longnecker et al.[27] identified the first carboxymethylated DNA adduct, N 7-carboxymethylguanine ( N 7-CMG) in pancreatic acinar cells treated with AS.
What is the melting point of azaserine?
Properties. Azaserine has a solubility of 50 mg/mL in water, a melting point of 146-162 °C, a vapor pressure of 1.53x10 −10 mmHg at 25 °C, and decomposes before melting.
Is azaserine a serine?
Azaserine is a naturally occurring serine derivative diazo compound with antineoplastic and antibiotic properties deriving from its action as a purinergic antagonist and structural similarity to glutamine.
Does azaserine inhibit hexosamine?
Azaserine inhibits the rate limiting step of the metabolic hexosamine pathway and irreversibly inhibits γ-glutamyltransferase by acting directly at the substrate-binding pocket. Independent of hexosamine pathway inhibition, azaserine has been demonstrated to protect against hyperglycemic endothelial damage by elevating serum concentrations of manganese-superoxide dismutase, directly reducing the concentration of reactive oxygen species.
