The new hepatitis
Hepatitis
Inflammatory condition of the liver.
Full Answer
Who pays for HCV medications?
High-Cost HCV Drugs in Medicaid: Final Report . 2 . Background . A Brief Epidemiology of Hepatitis C . Hepatitis C is a liver infection caused by the blood-borne hepatitis C virus (HCV), with seven distinct genotypes. 6,7. Transmission occurs mostly by percutaneous exposure, such as unsafe injection practices, needle-
How much does hepatitis C treatment cost?
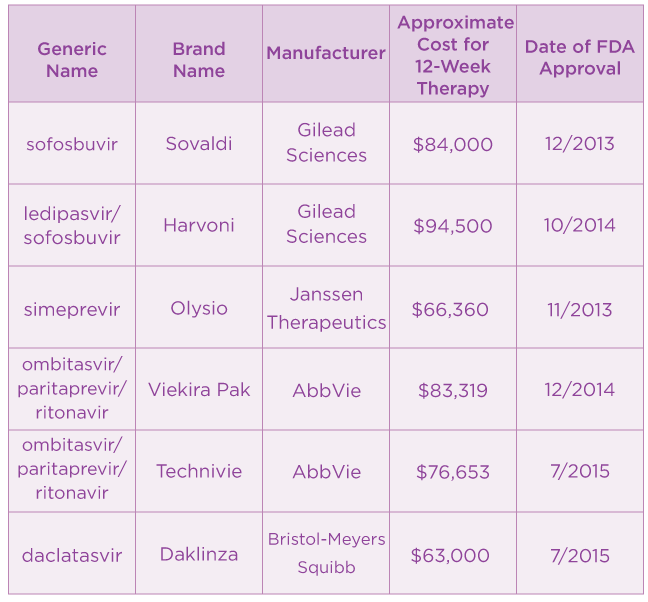
Nov 18, 2020 · Harvoni (ledipasvir/sofosbuvir) typically costs $94,500 for a 12-week treatment; Zepatier (elbasvir/grazoprevir) typically costs $54,600 for a 12-week treatment; Technivie (ombitasvir/paritaprevir/ritonavir) typically costs $76,653 for a 12-week treatment; Epculusa (sofosbuvir/velpatasvir) typically costs $94,500 for a 12-week treatment
What are the cure rates for HCV?
Jun 01, 2018 · The table below highlights the average cost of treatment for the combination DAAs currently available. Most of these drugs take at least 12 weeks to cure HCV, while the most recently approved drug ...
How much does Technivie (ombitasvir/paritaprevir/ritonavir) cost?
Just one pill of Sovaldi costs approximately $1,000. 26 This brings the total cost of the twelve-week treatment to $84,000. 27 Olysio has an estimated cost of $23,600 per month of treatment. 28 However, the treatment duration of Olysio is even longer than Sovaldi at twenty-four to forty-eight weeks. 29 While this is very expensive, the primary problem with the pricing is not the …

Does insurance cover hep C drugs?
Not all health insurance plans cover all prescribed medications for HCV treatment with few exceptions. Most insurers cover Sovaldi. It has an estimated copay of $75 to $175 per month. Check with your insurance provider to see what your individual coverage may entail.
How much does it cost to get rid of Hep C?
The Cost of Hepatitis C Treatment Harvoni cost even more -- $94,500 for a 12-week course, though some patients may be cured after only eight weeks, or $63,000. Gilead's newer offering, Epclusa, goes for just over $74,000. The gamechanger in the market may be Mavyret, which costs $26,500 for treatment.Sep 30, 2019
How much does Sovaldi cost in America?
Official Answer. The wholesale cost of Sovaldi is $1000 per 400mg tablet. A 12-week treatment course of Sovaldi costs around $84,000 and a 24-week course, $168,00.Apr 23, 2020
How much is Harvoni per pill?
Meet Harvoni, which launched in October and costs $1,125 per pill, or $94,500 for a 12-week course of treatment.Dec 19, 2014
Does Medicaid cover hep C treatment?
Although it is expensive, there are resources to help you pay for your hep C treatment. Medicaid and most insurance companies cover it.
Are hep C drugs expensive?
Hepatitis C drugs are pricey Antiviral drugs for hepatitis C are very effective, but they come at a steep cost. Just one Sovaldi pill costs $1,000. A full 12-week course of treatment with this drug costs $84,000.Feb 5, 2019
What are the side effects of Sovaldi?
Common side effects of Sovaldi include:fatigue,headache,nausea,insomnia,itching,anemia,weakness,rash,More items...
Does Sovaldi cure hep C?
Official Answer. Yes, Sovaldi does cure hepatitis C in most people when it is used in combination with at least one other hepatitis C treatment. A cure is defined as a sustained virologic response (SVR) for a certain period (usually 12 weeks) and is usually written as SVR12.Apr 24, 2020
Why is Sovaldi so cheap in India?
The newspaper says that current treatments for the disease in India run about $6,000 and require a 24- to 48-week course of injectables that come with serious side effects. So Sovaldi will be cheaper and easier to administer, with fewer side effects and the ability to cure many of the cases.
How can I get hep C treatment for free?
Patient assistance programs (PAPs) offer free hepatitis C drugs to lower-income people who are uninsured or underinsured, and who do not qualify for insurance programs such as Medicaid or Medicare.
How do you pay for hep C treatment?
Funding Resources Available to Hep C PatientsPharmaceutical Programs. ... The American Liver Foundation (ALF) ... NeedyMeds. ... Help-4-Hep. ... The HealthWell Foundation. ... The Pharmaceutical Research and Manufacturers of America (PhRMA) ... The Patient Access Network (PAN) Foundation. ... The Patient Advocate Foundation.Jun 9, 2021
Is hep C curable 2020?
Hepatitis C (hep C) infection used to be a lifelong condition for most people. Up to 50 percent of people may clear the hepatitis C virus (HCV) from their body without treatment. For everyone else, the infection becomes chronic. With advances in hep C treatment, most people can now be cured of HCV.
How much does hep C treatment cost?
Costs are changing, generally becoming cheaper thus these costs are approximate estimates and don’t list all the drugs now available for treatment. Quotes should be provided by your healthcare provider, commercial insurance provider, Medicaid, Medicare, VA, or other applicable healthcare providers/insurers: 1,2
What if I cannot afford treatment?
Many affected by hepatitis C don’t have insurance and therefore can’t absorb the high costs of treatment; Others can’t afford the co-pays required by insurance companies. In one study, it was estimated that 30% of those infected have no private insurance. 3 Another study estimated the rate at 65%.
Will my insurance pay for treatment?
For many who find out they are positive and next realize the cost of treatment, the big question is will my insurance pay for my hep C treatment. Unfortunately this is a complicated question with no clear answers. Insurance companies lack consistency about if and how much they will financially cover of the treatments.
Aftercare: Treatment Completion and Cured of Hep C
This article represents the opinions, thoughts, and experiences of the author; none of this content has been paid for by any advertiser. The HepatitisC.net team does not recommend or endorse any products or treatments discussed herein. Learn more about how we maintain editorial integrity here.
What is the new drug called for HCV?
Trusted Source. of people who take them, depending on the type of HCV infection and treatment exposure. These new drugs are called direct-acting antivirals (DAAs).
When was HCV approved?
The U.S. Food and Drug Administration (FDA) approved the first of these medications for HCV treatment in 2011. Several more medications have been approved since that time. Most of these individual drugs are effective for specific strains, or genotypes, of HCV.
What is the liver infection?
Hepatitis C is a viral infection that attacks the liver. Infection with hepatitis C can lead to serious liver disease, including cirrhosis and cancer. Hepatitis C virus (HCV) is transmitted by exposure to blood or other bodily fluids that contain HCV.
How many people die from hepatitis C each year?
Americans have chronic hepatitis C. About 19,000 of these people die each year from cirrhosis or liver cancer. Fortunately, recent advancements in the fight against this virus have changed the outlook for people with HCV. New drugs have transformed the disease from one that can, at best, be controlled to one that can be cured for most people who ...
What are the criteria for liver disease?
These criteria may be based on: the severity of liver disease. whether the person avoids alcohol and drug use. whether the drug’s prescribed by a doctor who specializes in liver diseases. the life expectancy of the person seeking treatment. whether less expensive treatments could be used first.
Is generic medicine cheaper than brand name?
It also means there are no generic versions of these drugs yet. Generics are typically much cheaper than brand- name versions. The FDA determines how long this period of exclusivity will last. During this time, the pharmaceutical companies have a lot of freedom in establishing prices.
Does insurance cover cirrhosis of the liver?
Payment restrictions. Based on your insurance provider, some companies will only pay for treatment if you have cirrhosis of the liver or bridging fibrosis , which is a thickening and scarring of the liver.
Why is it so expensive to get medicaid?
Many of the most costly drugs to Medicaid are so costly because they are frequently prescribed, including hydrocodone-acetaminophen, an opioid. While there are many medically necessary reasons to prescribe this drug, there is also a great deal of evidence to suggest overutilization of opioids.
How much did Medicaid spend on prescription drugs in 2014?
As a result, Medicaid prescription drug spending is sizeable: in 2014, Medicaid spent $27.3 billion on outpatient drugs. 5 Over the years, states have implemented an array of measures to control utilization and spending for prescription drugs. 6. In this issue brief, we look at which outpatient prescription drugs were most expensive ...
What is biologic drug?
A biologic is a drug that is derived from an animal or microorganism. It is more complex than traditional small-molecule drugs synthesized in a lab. 58 Because biologics are structurally very different from small molecule drugs and are approved through a different process, 59 there was not automatically a structure in place for generic approvals resulting in an absence of a generic market to commoditize biologic drugs. However, as part of the ACA, 60 biologics now have 12 years of regulatory exclusivity, 61 with an abbreviated pathway for the biosimilars, the biologic equivalent of a generic, now in place. Although biosimilars are expected to lower the price of the original biologic, they are not expected to lower it to that degree that generics lower the price of the original small-molecule brand drug. 62 In March 2015, the FDA approved its first biosimilar, Zarxio, and the drug launched the following September. 63
How long does it take for a drug to be approved by the FDA?
The FDA awards a regulatory exclusivity period of 3 or 5 years to brand drugs. 53 Regulatory exclusivity provides the manufacturer with a degree of market exclusivity, enabling them to price the drug accordingly and providing incentive for them to market it as a non-commodity, which includes naming the drug with appealing brand name. Alternatively, a manufacturer can obtain FDA approval for their drug by proving that it is bioequivalent to a brand drug, 54 skipping the long and expensive process of proving a drug is safe and effective. The FDA identifies these drugs as generic. 55 They cannot enter the market while the corresponding brand still has exclusivity. 56 Once generic drugs enter the market, the price of the drug usually falls due to competition.
What was the second most prescribed drug in 2014?
As a drug class, opioids were the second most prescribed drug group over the period of study and the most prescribed drug group in 2014 (data not shown). This high level of opioid prescriptions reflects the high level of use of opioids in the U.S. overall, which has been drawing more and more concern in recent years.
What are the most common prescription drugs for Medicaid?
Among the most commonly prescribed outpatient prescription drugs in Medicaid, the top five drugs are used for pain relief (hydrocodone-acetaminophen and ibuprofen), management of chronic illness (lisinopril and omeprazole), and antibiotics (amoxicillin) (see Appendix Table A3 ). However, these drugs are not necessarily among ...
How expensive is Sovaldi?
With its list price of $84,000 per treatment, the launch of the hepatitis C drug Sovaldi in December 2013 garnered the public’s and policymakers’ attention and brought into the spotlight the issue of high-cost prescription drugs in the U.S. Most Americans now believe that prescription drugs are too expensive. 1 With over 70 million beneficiaries, 2 the Medicaid program is larger than any other public or private insurer. 3 Many Medicaid beneficiaries have poorer health than enrollees in private coverage 4 and need prescription drugs to manage their medical conditions. As a result, Medicaid prescription drug spending is sizeable: in 2014, Medicaid spent $27.3 billion on outpatient drugs. 5 Over the years, states have implemented an array of measures to control utilization and spending for prescription drugs. 6
A Leading Killer
At a recent meeting on infectious diseases, Scott Holmberg, chief of the Epidemiology and Surveillance Branch at CDC’s Division of Viral Hepatitis, reported that in 2013, more Americans died of hepatitis C than from 59 other infectious diseases combined, including HIV and tuberculosis.
Discriminatory Restrictions?
Taylor’s study, published last summer, examined how state Medicaid agencies decide who will get Sovaldi, which is manufactured by the pharmaceutical company Gilead.
Stopping the Spread
Advocates for universal treatment also argue that treating patients sooner rather than later spares the health system the greater costs of treating liver cancer or undertaking transplants. It also prevents the spread of the infection to others.
