How does chlorination work in wastewater treatment?
Nearly every wastewater treatment facility uses chlorination to disinfect wastewater before the water is sent back out into the environment. The primary goal of chlorination is to disinfect the wastewater and remove any harmful pathogens that are present in the water.
What is chloramination of drinking water?
Chloramination is the treatment of drinking water with a chloramine disinfectant. As chloramines are more stable and last longer in water than chlorine, monochloramine is now used mostly as a secondary disinfectant because it provides more effective residual disinfection in the distribution system than chlorination alone.
What is the purpose of chloramination disinfection?
Chloramination disinfection is the practice of forming inorganic chloramines in water to reduce microbial concentrations to within acceptable limits. The chloramines -- monochloramine (NH2Cl), dichloramine (NHCl2) and nitrogen trichloride (NCl3) -- form when chlorine and ammonia are combined in water.
What is chlorination and why is it important?
The primary goal of chlorination is to disinfect the wastewater and remove any harmful pathogens that are present in the water. Once the wastewater has been properly treated, it can flow naturally into rivers, streams, and oceans without issue.
What are the benefits of water chlorination?
The benefits of chlorination are:Proven reduction of most bacteria and viruses in water.Residual protection against recontamination.Ease-of-use and acceptability.Proven reduction of diarrheal disease incidence.Scalability and low cost.
How does chlorination clean water?
Chlorine kills pathogens such as bacteria and viruses by breaking the chemical bonds in their molecules. Disinfectants that are used for this purpose consist of chlorine compounds which can exchange atoms with other compounds, such as enzymes in bacteria and other cells.
What happens when chlorine is added to water?
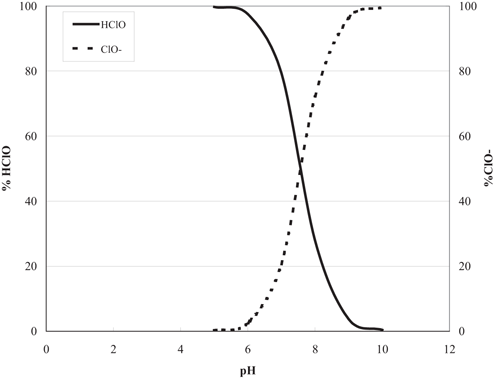
Chlorine will react in water to form hypochlorous acid, which can then dissociate into hydrogen and hypochlorite ions, according to Eqn (1). This reaction is very important, as the disinfecting power of HOCl, hypochlorous acid, is about 40–80 times that of OCl−, hypochlorite.
What is the advantage and disadvantage of chlorine?
When handled properly, according to manufacturers' directions, chlorine bleach is not only safe, but it also helps keep people healthy by killing harmful germs on surfaces. However, when chlorine bleach is misused – for example, mixed with ammonia or acids – the result can be harmful to your health.
Chlorination
The STP treats the wastewater coming from agriculture, sewage and industrial plants. The treatment process has four stages (pretreatment, primary, secondary and tertiary treatment).
Chlorine Compounds as Disinfectant
We can add chlorine into the tertiary system via chemical feed inlets. Three standard formulations of chlorine are available for chlorination:
Why is chlorine used in water treatment?
Therefore chlorine is used in their water treatment process. Chlorine continues to keep water safe after it leaves the Scottish Water treatment works and travels along the network of pipes on its way to homes and businesses. This helps to ensure that residents in Scotland receive high quality water. Download Scottish Water chlorine fact sheet.
Why is monochloramine used as a secondary disinfectant?
As chloramines are more stable and last longer in water than chlorine, monochloramine is now used mostly as a secondary disinfectant because it provides more effective residual disinfection in the distribution system than chlorination alone. It is used especially where disinfection by-product formation in the distribution system is exceedingly high ...
Where is monochloramine used?
It is used especially where disinfection by-product formation in the distribution system is exceedingly high if free chlorine is used as the secondary disinfectant. monochloramine may be produced on-site from ammonia and chlorine, or a preformed solution of monochloramine may be used.
Which disinfectant is the weakest?
Chloramine is the weakest disinfectant in this review but is more stable, and thus provides longer lasting residual disinfectant which can be an advantage for distribution systems. Due to its relatively low oxidation potential it is not thought form disinfection by-products at levels of concern. Like other chlorine types it is relatively ...
Is monochloramine a weak disinfectant?
As monochloramine is a weak disinfectant, especially against cysts and viruses, the contact times required for adequate primary disinfection are much longer and higher than with chlorine ...
Why is wastewater chlorination important?
The process of wastewater chlorination achieves one important goal: it disinfects the water and frees it of the harmful pathogens. This must be done, and it happens before the water runs off naturally into oceans, rivers and streams.
Why is chlorination needed in wastewater?
Chlorine needs to be put into wastewater to treat it and oxidize any contaminants it once held when in the sewage system. The chlorination wastewater treatment procedure creates byproducts in treated water.
Abstract
The reclamation and disinfection of waters impacted by human activities ( e.g., wastewater effluent discharges) are of growing interest for various applications but has been associated with the formation of toxic nitrogenous disinfection byproducts (N-DBPs).
Introduction
The disinfection of waters impacted by human activities ( e.g., agriculture or wastewater effluent discharges) has been associated with the formation of nitrogenous disinfection byproducts (N-DBPs) due to their enrichment in nitrogen-containing compounds ( e.g., ammonia or organic nitrogen such as amino acids and peptides) ( Bond et al., 2011, Westerhoff and Mash, 2002 ).
1. Materials and methods
1.1. Materials Analytical or laboratory grade reagents and were used without further purification. MilliQ water was produced with a Millipore system (18.2 MΩ/cm). Sodium hypochlorite (NaOCl, 5.65%–6%, Fisher Scientific) and ammonium chloride (Acros Organics, 99.6%) were used to prepare chloramine solutions.
2. Results and discussion
The EfOM extraction conditions and recovery for the three different batches of wastewater effluents (WW1, 2 and 3) are presented in Table 1, Table 2.
Acknowledgments
Research reported in this publication was supported by the King Abdullah University of Science and Technology (KAUST) Office of Competitive Research Funds, entitled “Nitrogenous Disinfection By-Products in Reclaimed Wastewater Effluents: Chemistry, Toxicity and Control Strategies”.
What is chloramination in water?
Chloramination disinfection is the practice of forming inorganic chloramines in water to reduce microbial concentrations to within acceptable limits. The chloramines -- monochloramine (NH2Cl), dichloramine (NHCl2) and nitrogen trichloride (NCl3) -- form when chlorine and ammonia are combined in water. Traditionally, treated wastewater, which contains ammonia, is disinfected by the addition of chlorine. In recent years, many drinking water facilities have converted to chloramination to disinfect potable water. Roughly 20% of all drinking water facilities in the United States now use chloramines as the residual disinfectant.
How is treated wastewater disinfected?
Traditionally, treated wastewater, which contains ammonia, is disinfected by the addition of chlorine. In recent years, many drinking water facilities have converted to chloramination to disinfect potable water.
What is the preferred disinfectant for drinking water?
For the chloramination of drinking water, monochloramine is the preferred disinfectant.
Does chlorine produce odors?
Formation of dichloramine and nitrogen trichloride is avoided, since more chlorine is consumed and the presence of these chloramines can produce odors or off-tastes. In treated wastewater, any organic nitrogen compounds present will form organic chloramines during chlorination.
Why is chlorination important in water treatment?
In order to combat waterborne diseases, different disinfection methods are used to inactivate pathogens. Along with other water treatment processes such as coagulation, sedimentation, and filtration, chlorination creates water that is safe for public consumption.
What is the purpose of adding chlorine to water?
The main objective of this chlorine addition is to disinfect the water and maintain chlorine residuals that will remain in the water as it travels through the distribution system.
What is the combination of free chorine and hypochlorite?
At lower pH levels, the hypochlorous acid will dominate. The combination of hypochlorous acid and hypochlorite ions makes up what is called ‘free chorine.’. Free chlorine has a high oxidation potential and is a more effective disinfectant than other forms of chlorine, such as chloramines.
What is chlorine breakpoint?
Residual Chlorine, Breakpoint. Any type of chlorine that is added to water during the treatment process will result in the formation of hypochlorous acid (HOCl) and hypochlorite ions (OCl-), which are the main disinfecting compounds in chlorinated water. More detail is provided later on in this fact sheet.
How is calcium hypochlorite made?
Calcium hypochlorite (CaOCl) is made up of the calcium salts of hypochlorous acid. It is produced by dissolving chlorine gas (Cl 2) into a solution of calcium oxide (CaO) and sodium hydroxide (NaOH). Calcium hypochlorite is a white, corrosive solid that comes either in tablet form or as a granular powder. Calcium hypochlorite is very stable, and when packaged properly, large amounts can be purchased and stored until needed. The chemical is very corrosive however, and thus requires proper handling when being used to treat water. Calcium hypochlorite needs to be stored in a dry area and kept away from organic materials. It cannot be stored near wood, cloth or petrol because the combination of calcium hypochlorite and organic material can create enough heat for an explosion. It must also be kept away from moisture because the tablets/granular powder readily adsorb moisture and will form (toxic) chlorine gas as a result. Calcium hypochlorite has a very strong chlorine odour – something that should be kept in mind when placing them in storage.
What happens after chlorine demand is met?
After the breakpoint, any additional chlorine added will result in a free chlorine residual proportional to the amount of chlorine added.
How much calcium hypochlorite is needed for water treatment?
Compared to the 1-16 mg/L required with chlorine gas, only 0.5-5 mg/L of calcium hypochlorite is required. When calcium hypochlorite is added to water, hypochlorite and calcium ions are produced.
