Inhibiting telomerase, an enzyme that rescues malignant cells from destruction by extending the protective caps on the ends of chromosomes, kills tumor cells but also triggers resistance pathways that allow cancer to survive and spread, scientists report in the Feb. 17 issue of Cell.
How could telomerase inhibitors be used to treat cancer?
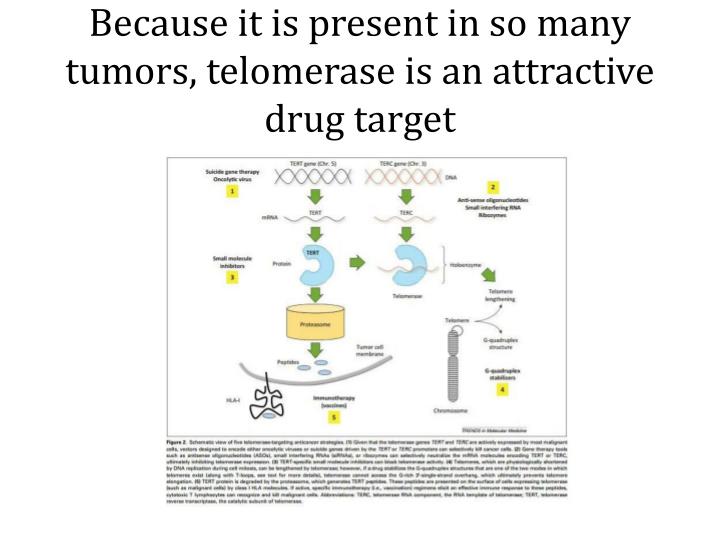
Due to telomerase inhibition, activity, or expression, these drugs might kill tumor cells by allowing telomeres to shrink or by provoking apoptosis. First of all, this process might have a chance to be cell-specific without serious side effects (Fig. 1).
How does telomerase work in cancer?
Cancer cells often avoid senescence or cell death by maintaining their telomeres despite repeated cell divisions. This is possible because the cancer cells activate an enzyme called telomerase, which adds genetic units onto the telomeres to prevent them from shortening to the point of causing senescence or cell death.
Can telomerase cure cancer?
Although the inhibition of telomerase may strip some cancers of their immortality, cancers are still viable and largely unaffected by the loss of telomerase. Furthermore, some cancers develop resistance to these inhibitors by means of ALT, rendering them totally ineffective [52].
How do telomeres prevent cancer?
"The DNA in telomeres shortens when cells divide, eventually halting cell division when the telomere reserve is depleted." New results from de Lange's lab provide the first evidence that telomere shortening helps prevent cancer in humans, likely because of its power to curtail cell division.
How does the telomerase work?
In egg and sperm cells, an enzyme called telomerase keeps adding more of the repeating sequence onto the end of DNA strands, so that the telomeres in these cells don't shorten. In other cells, telomerase is less active, leading to the gradual shortening of telomeres over time.
What happens if telomerase is blocked?
Without telomerase activity, these cells would become inactive, stop dividing and eventually die. Drugs that inhibit telomerase activity, or kill telomerase-producing cells, may potentially stop and kill cancer cells in their tracks.
Is telomerase active in cancer cells?
Cancer cells are characterized by high telomerase activity, which enables cells to divide indefinitely. Telomerase is active in 85–95% of cancers (3,4).
Do telomeres shorten in cancer cells?
While telomerase inhibition reveals that longer telomeres are more advantageous for cell survival, cancer cells often have paradoxically shorter telomeres compared with those found in the normal tissues.
Why are cancer cells immortal?
In most cases, cancer cells become immortal by invoking a genetic mutation that can trigger the production of an enzyme, known as telomerase, which prevents telomeres from shortening. Telomeres are important because they prevent DNA-containing chromosomes from damage or fusing with nearby chromosomes.
Are telomeres the key to aging and cancer?
Yet, each time a cell divides, the telomeres get shorter. When they get too short, the cell no longer can divide and becomes inactive or "senescent" or dies. This process is associated with aging, cancer and a higher risk of death.
Why would telomere shortening have a tumour suppressive effect?
The shortening of human telomeres has two opposing effects during cancer development. On the one hand, telomere shortening can exert a tumour-suppressive effect through the proliferation arrest induced by activating the kinases ATM and ATR at unprotected chromosome ends.
Is telomerase a tumor suppressor gene?
This review clarifies the difference between telomerase, which does not cause growth deregulation, and oncogenes, which do. It also addresses the concept of telomerase repression as a tumor suppressor mechanism early in life, with detrimental tissue degeneration and tumor-promoting consequences late in life.
How are telomeres related to cancer and aging?
Telomeres affect how our cells age. Once they lose a certain number of bases and become too short, the cell can no longer divide and be replicated. This inactivity or senescence leads to cell death (apoptosis) and the shortening of telomeres is associated with aging, cancer and an increased likelihood of death.
Can telomerase prevent normal cells from aging?
Every time cells divide, their telomeres shorten, which eventually prompts them to stop dividing and die. Telomerase prevents this decline in some kinds of cells, including stem cells, by lengthening telomeres, and the hope was that activating the enzyme could slow cellular ageing.
What is telomere therapy?
Regenerative and Anti-Aging Applications. Telomere attrition causes a wide range of age-related diseases. To this end, transfer of genes essential for telomere maintenance can be a promising strategy to combat these illnesses and restore the regenerative capacity of aging tissues.
What supplements lengthen telomeres?
Candidates include vitamin D, omega-3 fatty acids, and TA-65, a product that purportedly contains extracts of astragalus membranaceus, a plant-based compound that demonstrates immunomodulatory, anti-oxidative stress, and anti-aging effects, the latter of which are associated with longer telomeres.
What is telomerase in cancer?
Telomerase is expressed in more than 85% of cancer cells. Tumor cells with metastatic potential may have a high telomerase activity, allowing cells to escape from the inhibition of cell proliferation due to shortened telomeres. Human telomerase primarily consists of two main components: hTERT, a catalytic subunit, and hTR, an RNA template whose sequence is complimentary to the telomeric 5'-dTTAGGG-3' repeat. In humans, telomerase activity is typically restricted to renewing tissues, such as germ cells and stem cells, and is generally absent in normal cells. While hTR is constitutively expressed in most tissue types, hTERT expression levels are low enough that telomere length cannot be maintained, which sets a proliferative lifespan on normal cells. However, in the majority of cancers, telomerase maintains stable telomere length, thereby conferring cell immortality. Levels of hTERT mRNA are directly related to telomerase activity, thereby making it a more suitable therapeutic target than hTR. Recent data suggests that stabilization of telomeric G-quadruplexes may act to indirectly inhibit telomerase action by blocking hTR binding. Telomeric DNA has the propensity to spontaneously form intramolecular G-quadruplexes, four-stranded DNA secondary structures that are stabilized by the stacking of guanine residues in a planar arrangement. The functional roles of telomeric G-quadruplexes are not completely understood, but recent evidence suggests that they can stall the replication fork during DNA synthesis and inhibit telomere replication by preventing telomerase and related proteins from binding to the telomere. Long-term treatment with G-quadruplex stabilizers induces a gradual reduction in the length of the G-rich 3' end of the telomere without a reduction of the total telomere length, suggesting that telomerase activity is inhibited. However, inhibition of telomerase, either directly or indirectly, has shown only moderate success in cancer patients. Another promising approach of targeting the telomere is the use of guanine-rich oligonucleotides (GROs) homologous to the 3' telomere overhang sequence (T-oligos). T-oligos, particularly a specific 11-base oligonucleotide (5'-dGTTAGGGTTAG-3') called T11, have been shown to induce DNA damage responses (DDRs) such as senescence, apoptosis, and cell cycle arrest in numerous cancer cell types with minimal or no cytostatic effects in normal, non-transformed cells. As a result, T-oligos and other GROs are being investigated as prospective anticancer therapeutics. Interestingly, the DDRs induced by T-oligos in cancer cells are similar to the effects seen after progressive telomere degradation in normal cells. The loss of telomeres is an important tumor suppressor mechanism that is commonly absent in transformed malignant cells, and hence, T-oligos have garnered significant interest as a novel strategy to combat cancer. However, little is known about their mechanism of action. In this review, we discuss the current understanding of how T-oligos exert their antiproliferative effects in cancer cells and their role in inhibition of telomerase. We also discuss the current understanding of telomerase in cancer and various therapeutic targets related to the telomeres and telomerase.
What is the telomerase component of cancer?
Human telomerase primarily consists of two main components: hTERT, a cat …. Telomerase is expressed in more than 85% of cancer cells. Tumor cells with metastatic potential may have a high telomerase activity, allowing cells to escape from the inhibition of cell proliferation due to shortened telomeres. Human telomerase primarily consists of two ...
What is the role of T-oligos in cancer?
The loss of telomeres is an important tumor suppressor mechanism that is commonly absent in transformed malignant cells, and hence, T-oligos have garnered significant interest as a novel strategy to combat cancer. However, little is known about their mechanism of action.
Is telomerase a target for cancer?
However, in the majority of cancers, telomerase maintains stable telomere length, thereby conferring cell immortality. Levels of hTERT mRNA are directly related to telomerase activity, thereby making it a more suitable therapeutic target than hTR.
Is telomerase inhibitor clinically approved?
However, development of telomerase inhibitors has been challenging and thus far there are no clinically approved strategies exploiting this cancer target. The discovery of prevalent mutations in the TERT promoter region in many cancers and recent advances in telomerase biology has led to a renewed interest in targeting this enzyme.
Is telomerase reactivation a hallmark of cancer?
Telomere maintenance via telomerase reactivation is a nearly universal hallmark of cancer cells which enables replicative immortality. In contrast, telomerase activity is silenced in most adult somatic cells. Thus, telomerase represents an attractive target for highly selective cancer therapeutics. …
Is telomerase a target for cancer?
In contrast, telomerase activity is silenced in most adult somatic cells. Thus, telomerase represents an attractive target for highly selective cancer therapeut ics. However, development of telomerase inhibitors has been challenging and thus far there are no clinically approved strategies exploiting this cancer target. The discovery of prevalent mutations in the TERT promoter region in many cancers and recent advances in telomerase biology has led to a renewed interest in targeting this enzyme. Here we discuss recent efforts targeting telomerase, including immunotherapies and direct telomerase inhibitors, as well as emerging approaches such as targeting TERT gene expression driven by TERT promoter mutations. We also address some of the challenges to telomerase-directed therapies including potential therapeutic resistance and considerations for future therapeutic applications and translation into the clinical setting. Although much work remains to be done, effective strategies targeting telomerase will have a transformative impact for cancer therapy and the prospect of clinically effective drugs is boosted by recent advances in structural models of human telomerase.
What are the effects of telomerase?
In a series of experiments in a lymphoma mouse model, the team found: 1 Telomerase reactivation in malignant cells after genomic instability causes cancer progression. 2 Inhibiting telomerase caused tumor cell death but also led to alternative lengthening of telomeres (ALT) independent of telomerase. 3 ALT-positive cells increase both the expression and copy number of a gene called PGC-1ß, a key regulator of mitochondrial function, to compensate for mitochondrial and reactive oxygen species defense deficiencies. 4 Targeting PGC-1ß to weaken mitochondria function enhances anti-telomerase therapy.
How does telomerase extinction work?
Telomerase extinction works - at first. The team then took tumor cells from late-generation mice with activated telomerase - the aggressive tumors - and passaged them four times through groups of mice treated with either 4-OHT to trigger telomerase production or the control vehicle that leaves the enzyme off.
How long does telomerase reactivation last?
Telomerase reactivation causes aggressive cancer. Third- and fourth-generation mice with telomerase activated by 4-OHT had a median survival of 30 days and more frequent tumor infiltration to the spleen, kidney, liver, lung, bone marrow and brain than did control-treated mice, 70 percent of which lived beyond 50 days.
How many genes are in ALT positive tumors?
They found that ALT-positive tumors had different gene expression patterns - 891 genes with increased expression, 1,345 with decreased - compared to telomerase-positive tumors.
What is the main energy source for cancer cells?
In normal cells, power-generating mitochondria process fatty acids to produce ATP, a molecule that serves as the major energy source for the cell. Cancer cells generally rely more on sugar processing to generate energy. However, DePinho and colleagues note their genetic evidence suggests that mitochondria play a role supporting cancer cells.
What is the function of ALT cells?
ALT-positive cells increase both the expression and copy number of a gene called PGC-1ß, a key regulator of mitochondrial function, to compensate for mitochondrial and reactive oxygen species defense deficiencies.
What enzymes kill cancer cells?
Inhibiting telomerase, an enzyme that rescues malignant cells from destruction by extending the protective caps on the ends of chromosomes, kills tumor cells but also triggers resistance pathways that allow cancer to survive and spread, scientists report in the Feb. 17 issue of Cell. "Telomerase is overexpressed in many advanced cancers, ...
